OPEN-ACCESS PEER-REVIEWED
APPLICATION NOTE
Yun Wang Alelyunas, Gregory T. Roman, Jay Johnson, Catalin Doneanu, Mark D. Wrona
Waters Corporation, Milford, MA, USA.
Journal of Applied Bioanalysis. Vol.1. No.4. pages 128-135 (2015)
Published 15 October 2015. https://doi.org/10.17145/jab.15.021 | (ISSN 2405-710X)
Funding/Manuscript writing assistance:
The authors have no financial support or funding to report and also declare that no writing assistance was utilized in the production of this article.
Abstract
In this paper, high throughput analysis with 3 minute, rapid gradient conditions is described using the ionKey/MS™ System with an integrated ACQUITY UPLC® M-Class System and the Xevo® G2-XS QTof Mass Spectrometer. Extensive testing of representative small molecules and a peptide shows that the system is well-tolerated and exhibits excellent reproducibility and linear response. The iKey™ HSS T3 Separation Device used is robust, withstanding ~2200 injections of prepared human plasma with excellent peak shape and system pressure profile. A 99% solvent savings was realized when compared with an analytical system using a 2.1 mm column with flow rate ranging from 0.6 mL/min to 1.5 mL/min. These data, coupled with examples from the literature, illustrate that the ionKey/MS System with Xevo G2-XS QTof can be used as a full service platform for high throughput analysis and high sensitivity analysis to support all phases of drug discovery and development.
Introduction
High throughput LC-MS analysis typically refers to conditions of using a rapid gradient from 1 to 2 minutes followed by column washing, and column (re)equilibration, for a total gradient/cycle time of 2-5 minutes. This high throughput operation is important in drug discovery and bioanalysis settings due to the vast number of compounds that have gone through various in vitro and in vivo tests, where compound concentrations need to be quantified by LC-MS techniques. For these laboratories, short cycle times can be equally important to instrument sensitivity. Although microscale LC-MS is advantageous for high sensitivity analysis and reduced solvent consumption, it has not historically been used for high throughput analysis. This is largely due to previous limitations of long cycle time and poor reproducibility. These barriers have been overcome with the integrated and user-friendly ionKey/MS System [1]. In addition, the choice of MS platforms for bioanalysis is evolving, with high resolution mass spectrometry (HRMS) technologies gaining momentum, particularly in the area of biotherapeutic quantitation requiring high sensitivity [2]. This changing landscape can be best demonstrated through integration of the microfluidic iKey Separation Device with the Xevo G2-XS HRMS Mass Spectrometer. The signature attributes of this system include high sensitivity, high speed, solvent savings, and ease of use. Sensitivity and throughput of the system can be optimized by adjusting the system flow rate. To date, most applications have been carried out at or near a flow rate of 3 µL/min, with approximately 5-10 minute cycle times. The operating pressure using a 150 μm x 50 mm iKey is generally ~3000-3400 psi (200-227 bar) which is well under the iKey tolerance of 10000 psi. The ACQUITY UPLC M-Class Binary Pump can also deliver consistent flow rates up to 100 μL/min. The full capabilities for pressure and flow rate of the system have not yet been fully exploited. In this study, we investigated the performance of the system at higher flow rates and pressure conditions to perform high throughput analysis. System performance, including autosampler carryover, reproducibility, linear response, and iKey robustness were assessed using buspirone, a relatively polar small molecule drug, clopidogrel, a relatively non-polar compound, and oxytocin, a cyclic peptide hormone with a molecular weight of ~1000 Dalton.
Experimental
Samples description
Human plasma was treated via protein precipitation by the addition of acetonitrile (ACN) using a volume ratio of 3:1 (ACN:plasma). The solution was centrifuged at 13000 relative centrifugal force (RCF), and the supernatant was transferred to a new vial. The supernatant was then diluted with water containing 0.1% formic acid to a percentage of ACN that was specific for each of the three compounds. Test compounds: buspirone, buspirone-d8, clopidogrel, clopidogrel-d4, oxytocin, and oxytocin (Ile-13C6,15N) (Sigma Aldrich) were spiked into protein-precipitated human plasma. Final buspirone and clopidogrel samples contained 20% ACN, whereas the oxytocin samples contain 5% ACN. Other sample details are explained in the Results and Discussion section.
LC-MS conditions
The analytical LC-MS experiments were performed using the ionKey/MS System with the ACQUITY UPLC M-Class System, and the Xevo G2-XS QTof Mass Spectrometer. The ACQUITY UPLC M-Class System was configured with direct injection using an iKey HSS T3 Separation Device, 100Ǻ, 1.8 μm, 150 μm x 50 mm (P/N: 186007260). The iKey temperature was maintained at 65˚C. Flow rate was 7 μL/min. Mobile phase A consisted of 0.1% formic acid in H2O. Mobile phase B was 90% ACN/10% MeOH containing 0.1% formic acid. The injection volume was 1 μL. Sample manager temperature was 10˚C. Weak wash solvent was 10% ACN/90% H2O. Strong wash solvent was 25% ACN/25% IPA (isopropyl alcohol)/25% MeOH/25% H2O. Generic or compund-specific gradient conditions are described in the Results and Discussion section. The total run time was 3 minutes. Data was acquired using sensitivity mode with resolution >30000 FWHM under positive electrospray ionization. Acquisition range was 50-1200 m/z. Capillary voltage was 3.5 kV. Cone voltage was 60 V for the small molecules and 100 V for the peptide. The source temperature was 120°C, cone gas flow 50 L/hr, and nano gas flow was 0.1 L/hr. Scan times were either 0.1 s or 0.036 s and are detailed further in the Results and Discussion section. Tof MRM transitions for each of the compounds are as follows: buspirone, 386.3>122.0438, 394.3>122.0438 (IS), CE=30; clopidogrel, 322.1>212.0669, 326.1>216.0669 (IS), CE=16; and Oxytocin, 1007.4>1007.4454, 1014.4>1014.4454 (IS), CE=6. MassLynx® Software was used for data acquisition and TargetLynx™ Application Manager was used for data processing.
Results and discussion
Gradient condition and performance of the iKey
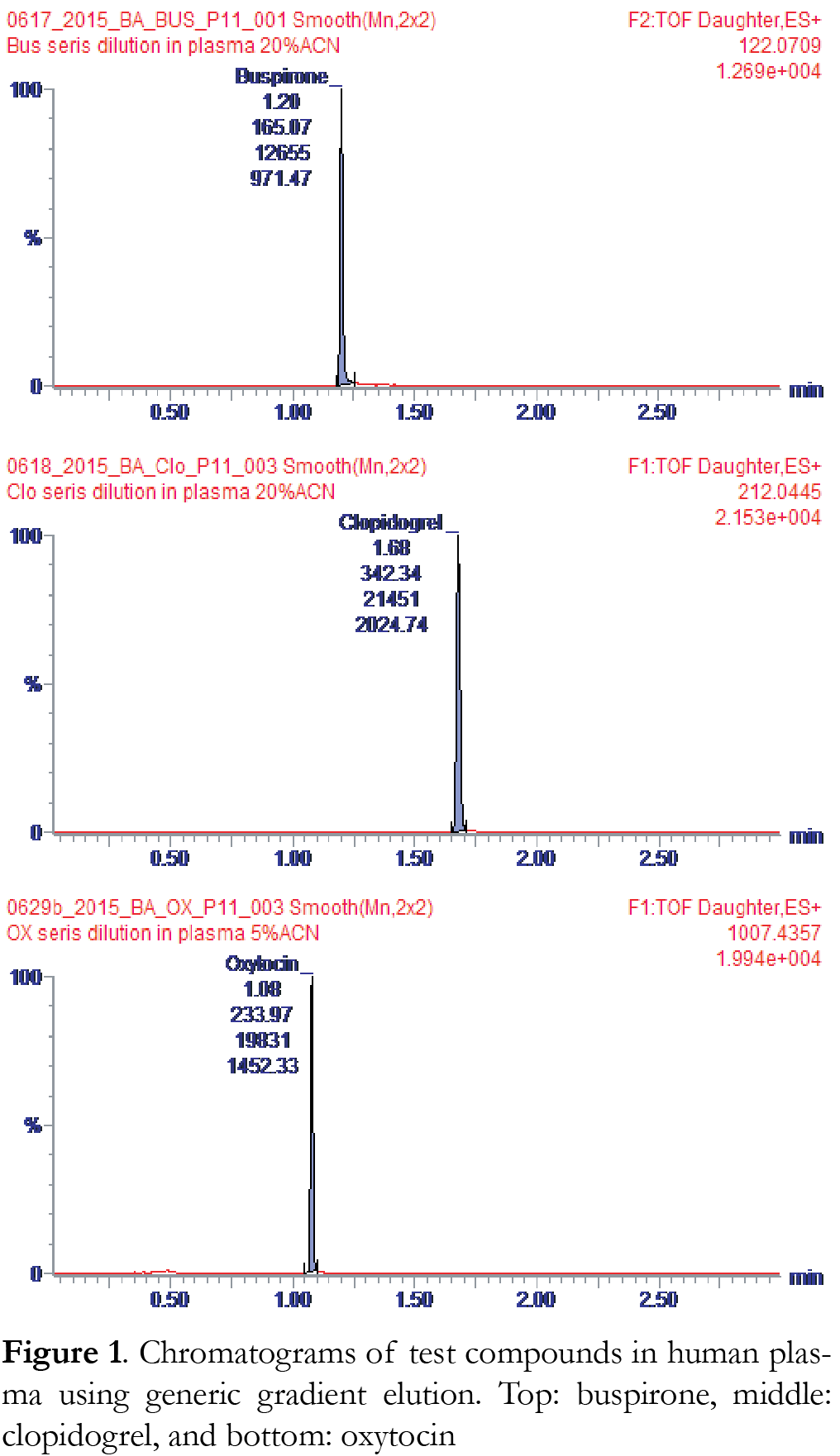
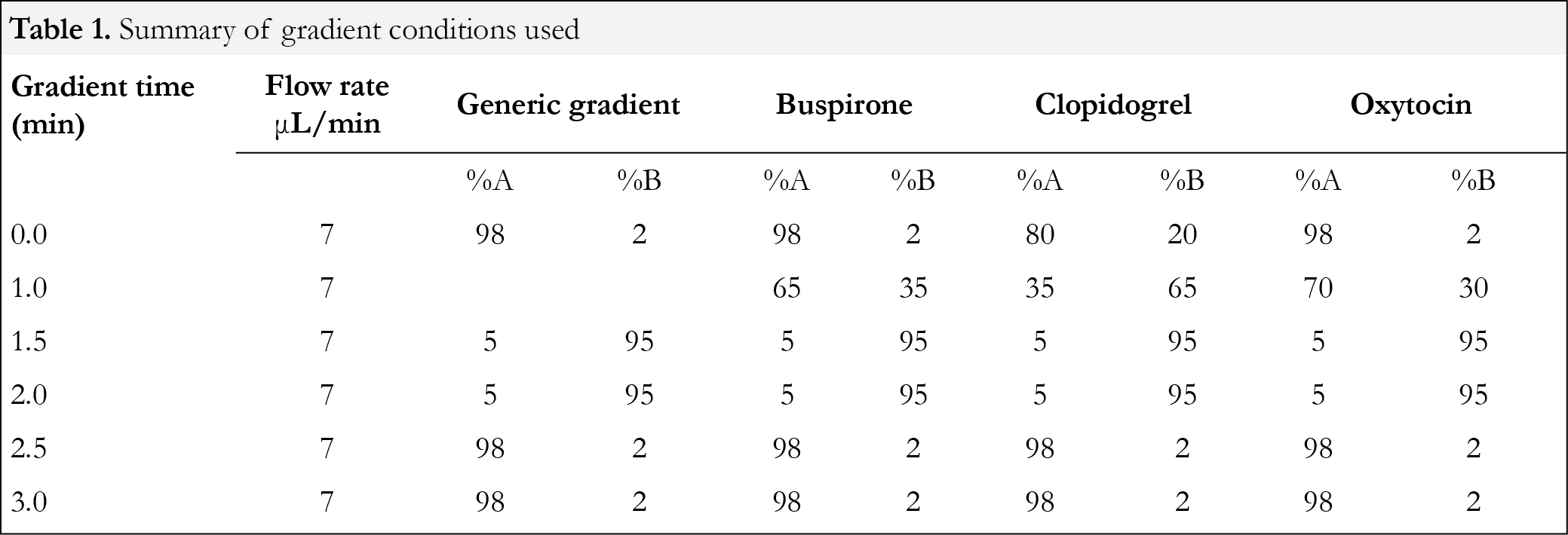
Generic or compound-specific conditions were used for each compound and are summarized in Table 1. For the generic condition, a ballistic gradient with increasing %B from 2% to 95% in 1.5 min was used. After holding at 95% B for 0.5 min, the %B was changed back to initial and held for 0.5 min (Table 1). The total gradient time was 3 minutes. Sample injection time was used to achieve complete iKey equilibration. The total cycle time, including sample injection, was approximately 4 minutes. Chromatograms for each of the three compounds studied are shown in Figure 1. For the compound-specific gradient conditions, a brief method development was carried out where the percent mobile phase was adjusted for the first minute to enable the compound eluting at approximately 1.5 min. The subsequent ramping to 95 %B, holding and return back to the initial condition were the same as the generic gradient. Using a 1 μL sample loop, the system’s theoretical delay volume was calculated to be 3.65 μL. The increase of flow rate to 7 μL/min corresponds to a theoretical delay time of 0.52 min. The performance of BEH and HSS T3 iKeys were tested. Both iKeys are packed with C18 for reversed phase applications.
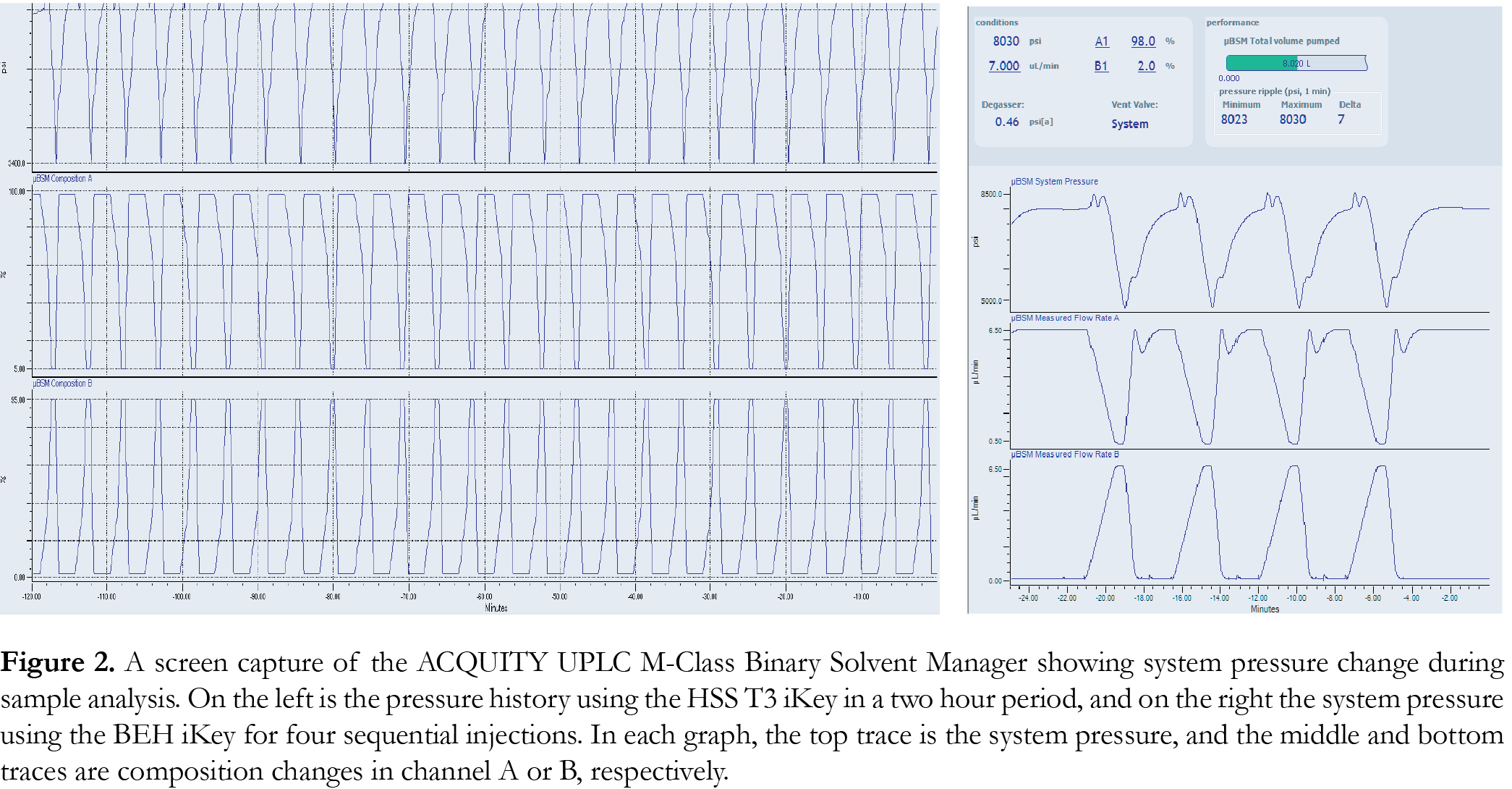
The HSS T3 iKey is packed with high strength silica with tri-functional C18 alkyl phase bonding, which enables the column to withstand high pressure and promote polar compound retention and aqueous phase compatibility. The pressure traces shown in Figure 2 show that both iKeys yielded highly reproducible profiles. The pressure for the BEH iKey was found to cycle between 4500 and 8500 psi (300-567 bar), and the HSS T3 between 3400 and 6400 psi (227-426 bar). The iKey packed with 1.8 µm HSS T3 particles exhibited a lower maximum system pressure profile vs. the 1.7 µm BEH particles and was used in subsequent studies reported in this application note.Optimisation of the limit of quantification
The higher limit of quantification (HLOQ) and LLOQ are affected by the coefficient of variation (CV) of the immunoassay. The HLOQ can easily be circumvented by dilution of the samples. However, the LLOQ is primarily determined by the sensitivity of the method and, therefore, harder to reduce. To lower the LLOQ the main parameter to be optimised was the sample volume. The sample volume was increased from the standard 3 µL to 18 µL to increase the total amount of tobramycin to be measured in the sample and thus, the sensitivity of the method. Accordingly the HLOQ is expected to be lower. By varying the sample volume, the measuring range of the Syva® Emit® 2000 Tobramycin Assay, in which it can accurately quantitate tobramycin concentrations, can be significantly increased. To demonstrate that increasing the sample volume does not have a profound effect on the sensitivity of the assay, the method was extensively validated.
System carryover
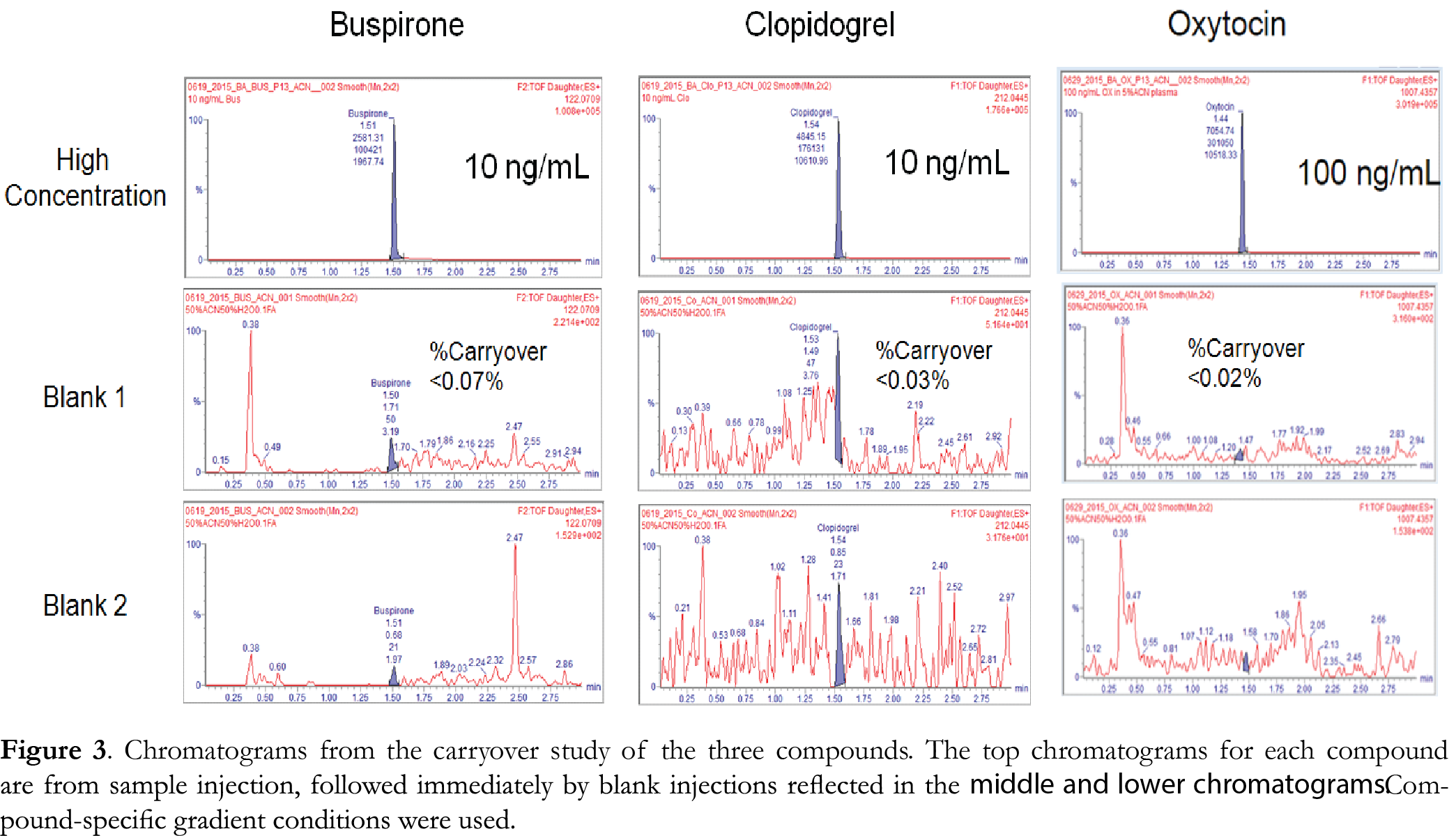
System carryover was measured by injecting a highly concentrated solution of each compound followed by blank solutions consisting of 50% ACN/50% H2O. The high concentration used was 10 ng/mL for buspirone and clopidogrel, and 100 ng/mL for oxytocin. These concentrations are near the saturation level of the Xevo G2-XS QTof for these compounds. Figure 3 shows chromatograms of the sample and the two blanks injected immediately afterwards. Percentage carryover was calculated based on the peak area at the retention time of the sample. The % carryover calculated for the blank injection immediately after the sample is 0.07% for buspirone, 0.03% for clopidogrel, and 0.02% for oxytocin. These results suggest that there is minimal or no sample carryover using the ionKey/MS with Xevo G2-XS under these conditions.
Figures and Tables
[Click to enlarge]
Reproducibility
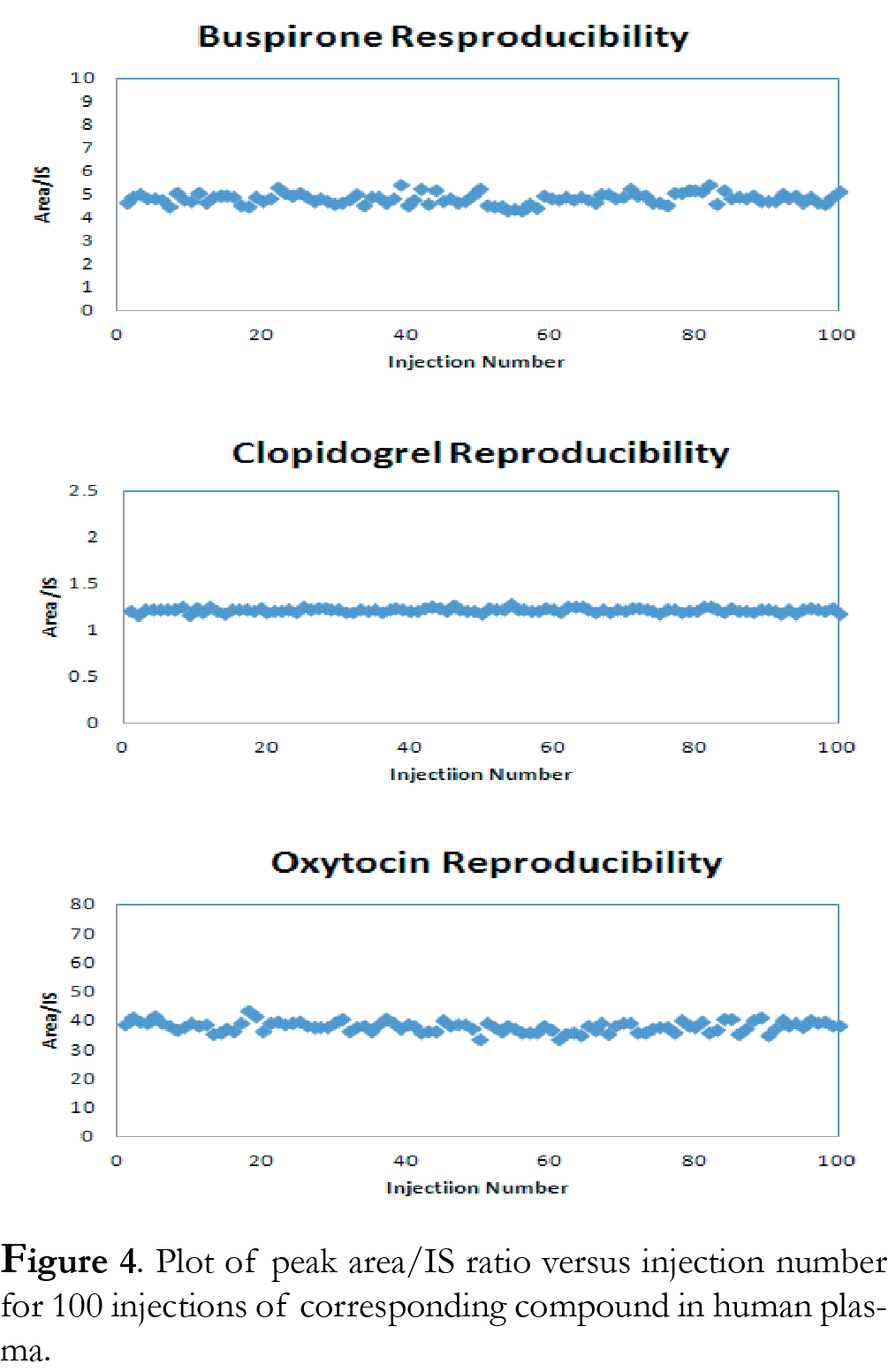
The reproducibility was tested by injecting the same sample and internal standard solution 100 times using either generic or compound-specific gradient conditions. Figure 4 shows a representative plot of peak area versus injection numbers. Percent RSD was calculated as 4.8% for buspirone, 1.7% for clopidogrel, and 4.6% for oxytocin. The data suggest good reproducibility for the ionKey/MS System under high throughput analysis conditions.
Linear response
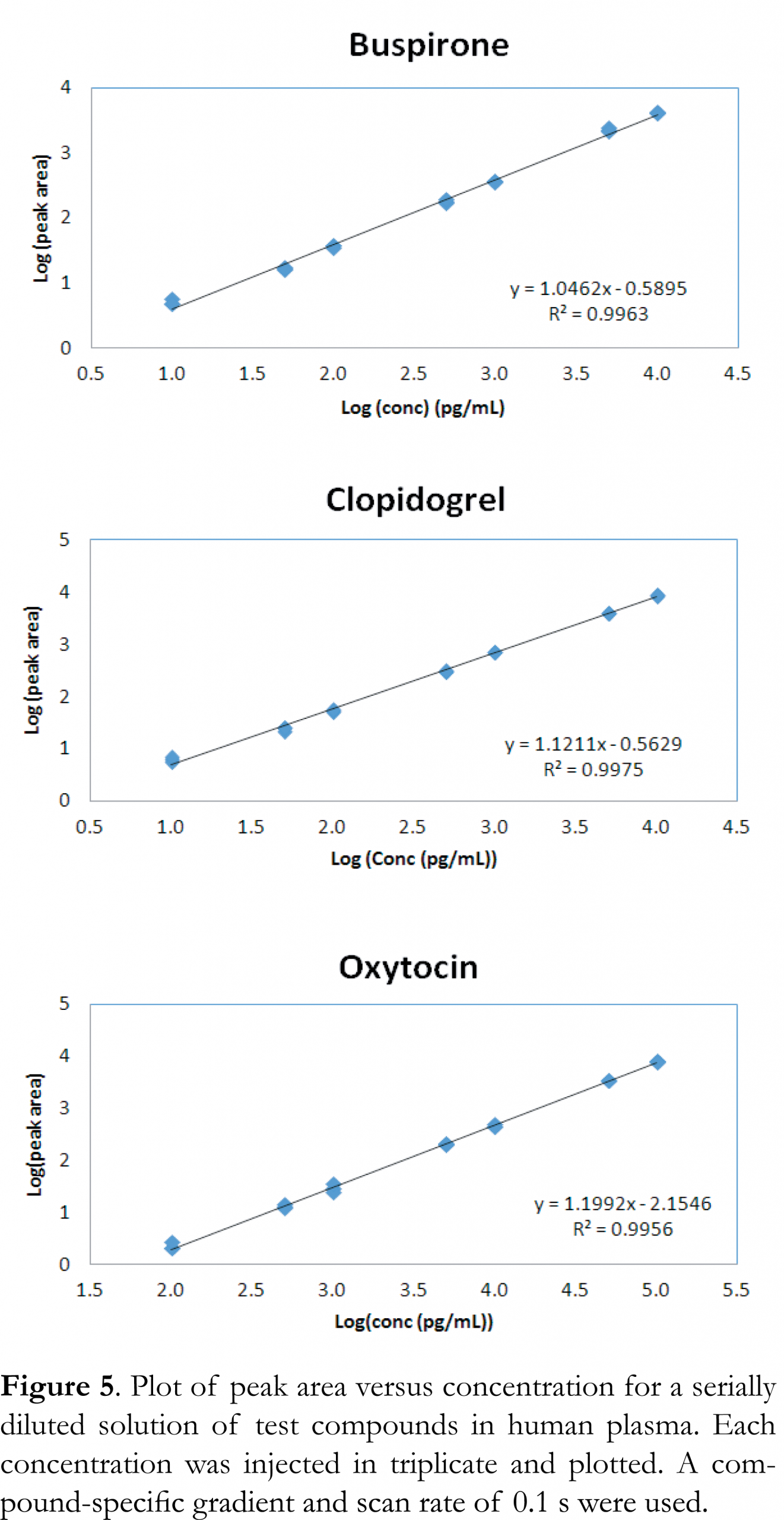
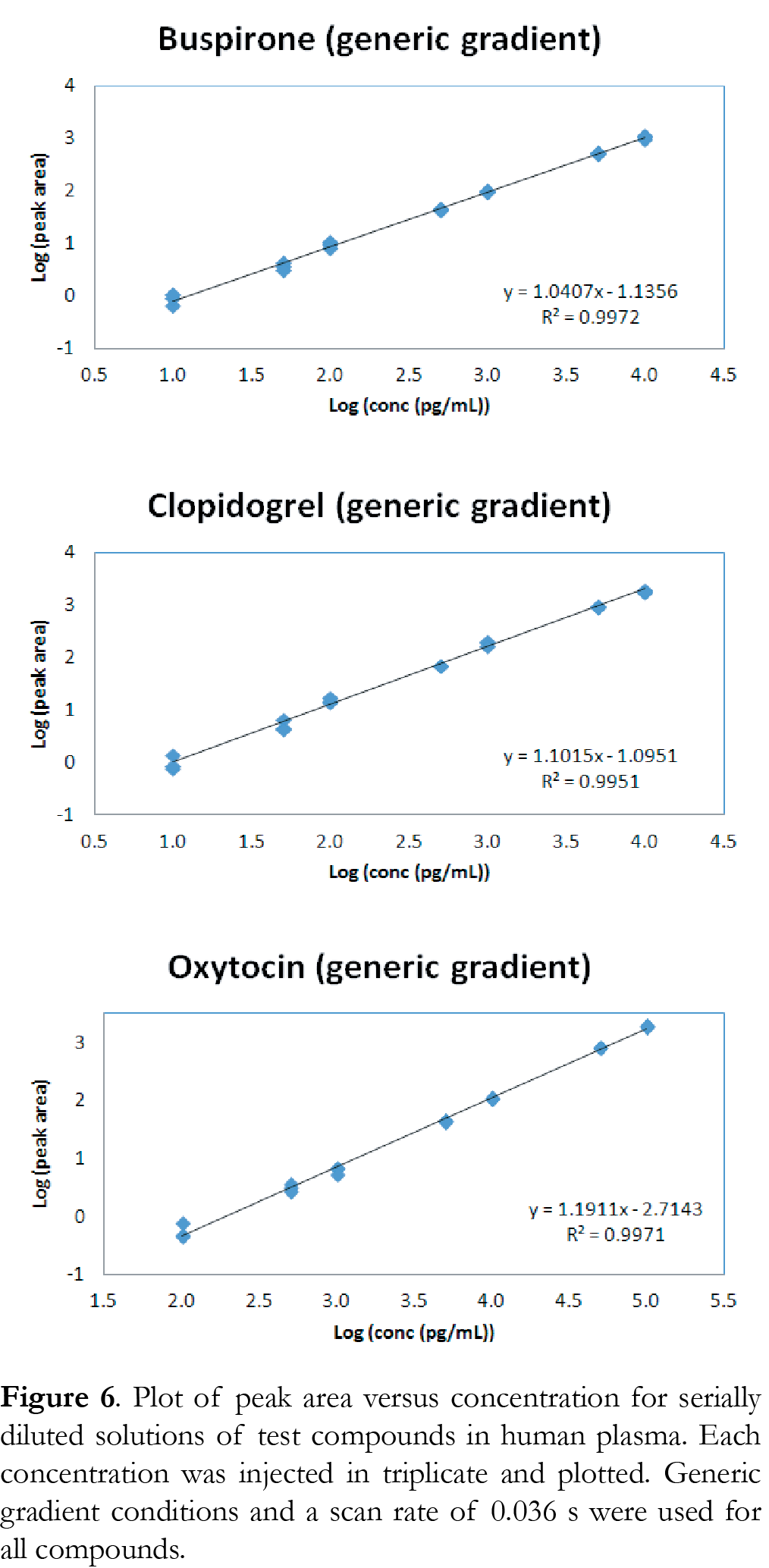
The linear response was determined for serial diluted solutions in human plasma ranging from 1 pg/mL to 10 ng/mL for buspirone and clopidogrel, and from 10 pg/mL to 100 ng/mL for oxytocin. Each compound solution was injected in triplicate and analyzed using both generic and compound-specific conditions. The Tof MRM scan rate was 0.1 second for the compound-specific gradient, and 0.036 seconds for the generic gradient. The faster scan time at the generic gradient condition ensured that a minimum of 10 data points across the peak were collected for both the compound and internal standard when they eluted early with narrower peak width in the gradient time window. Figure 5 shows the linear response curve using compound-specific conditions. Figure 6 shows the linear response curve using generic gradient conditions. Results show excellent linear response under all conditions and scan rates, with R2 >0.995 for all three compounds tested. For buspirone and clopidogrel, the linear range extended from 10 pg/mL to 10 ng/mL, and for oxytocin, the range was 100 pg/mL to 100 ng/mL using both generic and compound-specific gradients. The data show that the ionKey/MS System with Xevo G2-XS QTof can be used to perform quantitative analysis under high throughput conditions.
Effect of flow rate on MS response
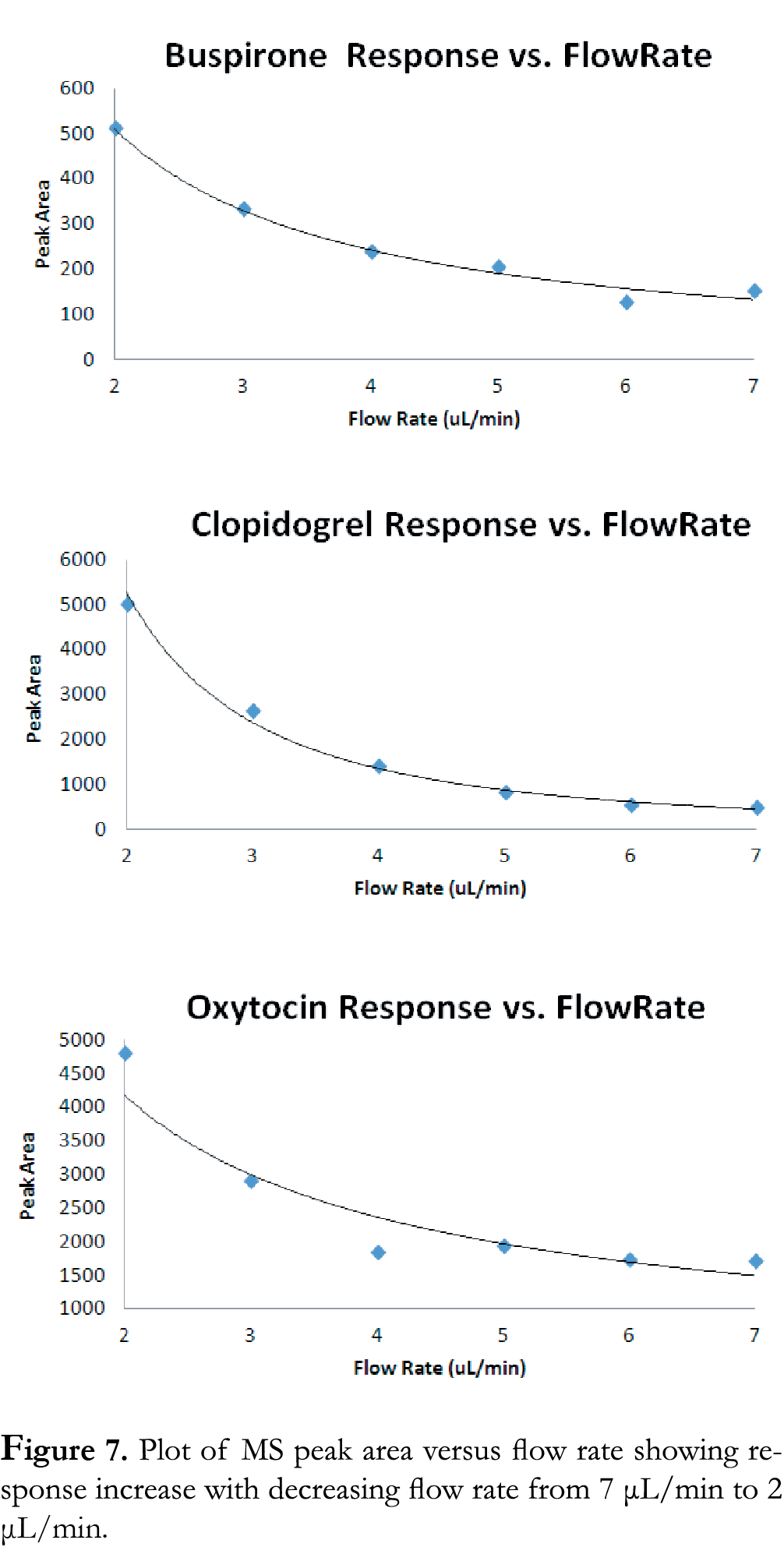
Available literature suggests that MS response is a function of flow rate in micro flow systems. In general, MS response increases with decreasing flow rate as the result of reduced droplet size and increases in ionization efficiency and MS sampling [3]. This effect is also measured in the present study by collecting data at flow rates from 7 μL/min to 2 μL/min. Table 2 is a summary of the gradient times for each of the flow rates used. The time for each segment of the gradient was scaled based on column volume. Figure 7 is a plot of MS response versus flow rate. Results show that with flow rate decreasing from 7 μL/min to 2 μL/min, there is an exponential increase in MS response for all three compounds used in the present study, which is consistent with the reported literature [3]. This provides a facile path to transition from high throughput analysis to extreme high sensitivity analysis by simply modulating flow rate. Incorporating trap-and-elute ionKey configurations has also been employed to further enhance the overall system sensitivity and has been described elsewhere [4].
iKey robustness
The data presented here were collected using a single HSS T3 iKey, and by the end of the study ~2200 injections of human plasma had been injected, most of which were collected at the 7 μL/min flow rate. The iKey remained viable beyond these ~2200 injections. Figure 8 is a screen capture of the iKey history as monitored and stored by MassLynx Software. The history shows that with the first injections of the iKey, the maximum pressure is approximately 6400-6500 psi and is consistent to the end of the study. The peak shape also remains excellent.
Solvent usage
Solvent consumption and cost continues to be a concern in DMPK discovery labs. Due to the large amount of solvent used, many labs have also invested in expensive systems that can dispense solvent from larger solvent containers which need to follow strict safety guidelines. The 7 μL/min flow rate used in the present study represents a 98.8% to 99.5% solvent savings, assuming analytical scale chromatography with a flow rate ranging from 0.6 mL/min to 1.5 mL/min. To put this into perspective, Figure 9 shows that consuming 1 L of solvent on the ionKey/MS System at a flow rate of 7 μL/min is equivalent to consuming thirty-six 1-gallon bottles at a flow rate of 1 mL/min. Lower solvent requirements also translates to reduced time and labor required for preparation. The reduced solvent usage and consequential reduced waste disposal is increasingly important in making companies and laboratories more “green” and environmental friendly.
Conclusion
The ionKey/MS System with Xevo G2-XS QTof HRMS is a high performance platform that can be used for highly sensitive detection of small molecules and peptides (and other biomolecules). The present application shows that the ionKey/MS can also be operated under high throughput conditions with a 3 minute full gradient and a 7 μL/min flow rate. Using representative small molecules and a peptide, extensive testing of the platform shows that it is well-suited for routine analysis with high reproducibility, excellent linear response and minimal sample carryover. The HSS T3 iKey is also shown to be robust under high throughput conditions, after ~2200 injections of human plasma, with minimal impact on the system’s maximum pressure or peak shape. In summary, the present data, coupled with the growing body of literature on the utility and robustness of next generation microfluidics, suggests the ionKey/MS with Xevo G2-XS QTof can be used as a full service platform for high throughput and high sensitivity analysis to support all phases of drug discovery and development.
Acknowledgement
We’d like to acknowledge the following from Waters Corp for the review of the manuscript: Kelly Doering, Pete Claise, Nigel Ewing, Larry Hines, and Sylvia Vollering.
References
1. Rainville PD, Langridge JI, Wrona MD, Wilson ID, Plumb RS. Integration of microfluidic LC with HRMS for the analysis of analytes in biofluids: past, present and future. Bioanalysis. 7(11), 1397-411 (2015).
2. Ramanathan R, Jemal M, Ramagiri, Xia SY, WG Humpreys WG, T Olah, WA Korfmacher. It is time for a paradigm shift in drug discovery bioanalysis: From SRM to HRMS. J Mass spectrom. 46(6), 595-601 (2011).
3. Murphy JP, Johnson J, Rainville PD. Enhancing Mass Spectrometry Sensitivity by Reducing Chromatographic Flow Rates with ionKey/MS, Waters White Paper (2014). (P/N 720004967EN).
4. Alelyunas YW, Wrona MD, Murphy J, Doneanu A, Roman G, Rainville P. Exploring Extra Sensitivity Using ionKey/MS with the Xevo G2-XS Q-Tof HRMS for Small Molecule Pharmaceutical Analysis in Human Plasma. Waters Application Note (2015). (P/N 720005371EN)
All site content, except where otherwise noted, is licensed under a Creative Commons Attribution 4.0 License