OPEN-ACCESS PEER-REVIEWED
REVIEW ARTICLE
Ganesh S. Moorthy1,2*, Kevin J. Downes1,3,4, Nicole R. Zane1, Athena F. Zuppa1,2
1Center for Clinical Pharmacology, Children’s Hospital of Philadelphia, Philadelphia, USA. 2Department of Anesthesiology and Critical Care Medicine, Children’s Hospital of Philadelphia, Philadelphia, USA. 3Division of Infectious Diseases, Children’s Hospital of Philadelphia, Philadelphia, USA. 4Department of Pediatrics, Children’s Hospital of Philadelphia, and Perelman School of Medicine, University of Pennsylvania, Philadelphia, USA.
Journal of Applied Bioanalysis. Vol.4. No.5. pages 144-156 (2018)
Published 15 December 2018. https://doi.org/10.17145/jab.18.019 | (ISSN 2405-710X)
*Correspondence:
Moorthy GS . Center for Clinical Pharmacology, The Children’s Hospital of Philadelphia, 3501 Civic Center Blvd, CTRB 4012, Philadelphia, PA 19104, USA.
Phone: +1 215 590 0142.
Citation:
Moorthy GS, Downes KJ, Zane NR, Zuppa AF. Liquid Chromatography-Tandem Mass Spectrometry Assays for Therapeutic Drug Monitoring of Cefepime. J Appl Bioanal 4(5), 144-156 (2018).
Editor: Dr. Jan-Willem Alffenaar, University of Groningen, University Medical Center Groningen, Department of Clinical Pharmacy and Pharmacology, Groningen, The Netherlands.
Open-access and Copyright:
©2018 Moorthy GS et al. This article is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Funding/Manuscript writing assistance:
The authors have no financial support or funding to report and they also declare that no writing assistance was utilized in the production of this article.
Competing interest:
The authors have declared that no competing interest exists.
Article history:
Received 08 October 2018, Revised 29 November 2018, Accepted 02 December 2018.
Abstract
With the development and growth of multi-drug resistance organisms, therapeutic drug monitoring (TDM) has become an increasingly common tool to assure the efficacy and safety of antimicrobial therapy. The requirement for TDM has not routinely extended to cephalosporins, such as cefepime, due to their wide therapeutic window. However, the implementation of TDM for cefepime is becoming increasingly important as many Gram-negative bacteria have adapted mechanisms that increase resistance to antimicrobials and therefore require higher concentrations of cefepime for bacterial eradication. Additionally, pharmacokinetic variability and difficulty in treating patients, especially those who are critically ill or those with renal dysfunction, necessitate TDM to ensure optimal cefepime concentrations.
This review aims to assess and evaluate the techniques currently utilized to quantify cefepime in both plasma and serum in terms of usefulness for feasible and readily adaptable bedside cefepime TDM.
Keywords
Cefepime, therapeutic drug monitoring, liquid chromatography, tandem mass spectrometry.
Introduction
Cefepime is a 4th-generation cephalosporin with activity against a range of facultative Gram-positive and Gram-negative bacteria [1]. Due to its broad spectrum of activity, it is frequently used as empiric antibiotic coverage in patients suspected to have serious bacterial infections, such as critically ill and immunocompromised patients. It is also administered as definitive treatment for infections caused by Gram-negative bacilli resistant to narrower spectrum antibiotic agents, including Enterobacteriaceae that produce AmpC β-lactamases [2] and many strains of Pseudomonas aeruginosa [3]. As a result, cefepime has become an important antimicrobial agent in the treatment of serious community- and hospital-acquired bacterial infections. The pharmacokinetics (PK) of cefepime have been well-characterized. It is renally eliminated with greater than 80% of doses excreted unchanged in the urine [4], and roughly 16% of total serum concentrations are bound to plasma proteins [5]. Cefepime displays linear pharmacokinetics over a range of doses [4, 6]. In patients with normal renal function, cefepime has an elimination half-life of 2-3 hours in adults [4], 1.5-1.9 hours in infants and children [6], and 4.9 hours in neonates [7]. In patients with renal failure and in those receiving continuous renal replacement therapy, total body clearance is delayed [8, 9], necessitating the use of longer dosing intervals or lower total daily doses.
Cefepime demonstrates time-dependent bactericidal activity in which its efficacy is defined by the fraction of time for which the free (unbound) concentration is maintained above the minimum inhibitory concentration (MIC) of the bacteria being treated. The classic pharmacodynamic (PD) target that is associated with improved clinical outcomes for cefepime has been maintenance of drug concentrations above the MIC (fT>MIC) for ≥60% of dosing interval [10]. However, more recent data have suggested that more aggressive PD targets, maintaining drug concentrations above the MIC for the entire dosing interval (100% fT>MIC), should be used to ensure optimal clinical and microbiological outcomes [11,12]. Successful treatment of harder to treat infections, such as Gram-negative pneumonia, may require the administration of doses that maintain free serum concentrations at least 2-fold higher than the MIC for the entire dosing interval (100% fT>2xMIC, or fCmin>2 xMIC) [13]. Meanwhile, in vitro studies have demonstrated that suppression of resistance selection during therapy occurs with an even more robust PD target of fCmin>3.8xMIC [14]. These latter data are consistent within vitro time-kill studies for Pseudomonas aeruginosa that show increased bacterial killing for β-lactam agents at concentrations up to 4 times the MIC [15]; based on these time-kill data, some studies have used fT>4xMIC as the optimal PD target for β-lactam antibiotics [12].
In 2014, the Clinical and Laboratory Standards Institute (CLSI) revised cefepime interpretive criteria (breakpoints) for the treatment of Enterobacteriaceae based on data showing clinical failures and low probability of target attainment using standard cefepime dosing in adults infected with Gram-negative bacteria with an MIC of 4 and 8 µg/mL [16, 17]. To optimize the administration of cefepime, CLSI employed the designation of “susceptible-dose dependent” (SDD) for isolates with an MIC of 4 and 8 µg/mL [17]. This change was made to signal that dosing regimens that result in higher drug exposures (i.e. higher doses or more frequent dosing) be administered to give the highest probability of adequate coverage of these isolates [17]. Instead of using the term “intermediate”, which is often clinically interpreted as “resistant,” the SDD designation was implemented to encourage the administration of cefepime at high doses rather than discourage its use for treatment of less susceptible isolates. The SDD designation does not apply to Pseudomonas aeruginosa and other non-Enterobacteriaceae Gram-negatives since the approved dosing regimen for treatment of these organisms differ [17].
There is accumulating evidence in critically ill adults that standard β-lactam dosing by intermittent infusion, including for cefepime, often produces inadequate antibiotic concentrations contributing to suboptimal outcomes [12,18-19]. Pediatric studies also have found that traditional dosing via intermittent infusion may be inadequate for less susceptible Gram-negative pathogens [20]. As a result, there is increased acceptance that administration of β-lactam agents as an extended or continuous infusion maximizes the likelihood of PD target attainment in critically ill patients [21]. The administration of cefepime as a prolonged infusion significantly increases the probability of PD target attainment compared to intermittent infusion, even for isolates in the SDD range [20,22]. A recent meta-analysis of 6 randomized controlled trials (RCTs) and 4 observational studies found that prolonged infusion of meropenem was associated with higher clinical success rate (odds ratio 2.10, 95% CI 1.31-3.38) and lower mortality (risk ratio 0.66, 95% CI 0.50-0.88) in adult patients with severe infections compared to intermittent infusion [23]. Similarly, in a meta-analysis of 13 RCTs comparing continuous infusion versus intermittent bolus of β-lactam agents in critically adult patients with respiratory infections, continuous infusion was associated with higher cure rates (risk ratio 1.18, 95% CI 1.07-1.30) [24]. Although data specific to cefepime are limited, there is mounting evidence that the use of prolonged infusions improves the likelihood of PD target attainment, which is especially important in patients with critical illness, serious bacterial infections, and infections caused by isolates with decreased susceptibility to the β-lactam agent being administered.
The production of β-lactamases is the most frequent and important mechanism of resistance to β-lactam antibiotics among Gram-negative bacteria. Extended-spectrum β-lactamases (ESBLs), carbapenemases, and AmpC β-lactamases have the capacity to hydrolyze drugs in this class with SHV-, OXA- and CTX-M-type enzymes being most prominent among Pseudomonas aeruginosa and Enterobacteraciae [25,26]. CTX-M-15 is the most widely distributed CTX-M-type ESBL and isolates producing this enzyme have higher MICs to cefepime than other ESBLs [27]. As noted above, maintenance of drug concentrations 4-fold above the MIC for the entire dosing interval decreases selection of resistance during cefepime therapy in a hollow-fiber infection model [14]. Although in vivo studies are needed to validate this target, optimized antibiotic exposures can prevent, or at least delay, the development of resistance among Gram-negative bacteria during treatment, particularly in high-burden infections such as pneumonia or undrained, intra-abdominal abscesses.
While administration of large doses can facilitate achievement of higher drug concentrations, cefepime is associated with dose-dependent toxicity particularly neurotoxicity [28-31], especially in patients with renal dysfunction, limiting the administration of overly large doses. In a single-center retrospective study of adult patient receiving cefepime who underwent therapeutic drug monitoring (TDM), a neurologic event considered possibly related to cefepime occurred in 11% of 93 patients [29]; patients with Cmin > 20 mg/L had a 5-fold higher risk for neurologic events (OR 5.1, 95% CI 1.3-19.8) and those with a Cmin > 40 mg/L had a 9-fold higher risk (OR 9.4, 95% CI 2.2-39.5). Decreased renal function was significantly associated with neurotoxicity [29]. Similarly, in a study of adult patients receiving cefepime for febrile neutropenia, high cefepime concentrations were an independent predictor of neurological toxicity with a 50% probability of toxicity at Cmin > 22mg/L [30]. Patients with acute or chronic renal dysfunction may have reduced clearance of cefepime, depending on the degree of glomerular filtration impairment, and are at increased risk of exposure-dependent neurotoxicity.
It is crucial to individualize cefepime dosing in order to achieve concentrations that optimize efficacy, limit selection of resistant bacteria, and avoid toxicity. This is especially true for critically ill patients and those with Gram-negative infections caused by less susceptible isolates who are at high risk of suboptimal drug exposures and resultant clinical failure, as well as patients with renal dysfunction at higher risk for cefepime-induced neurotoxicity. Monitoring of blood concentrations is particularly important in order to guide dosing that will achieve targeted serum drug concentrations. Although data demonstrating improved clinical outcomes with TDM for β-lactam agents are limited, TDM has been shown to improve PD target attainment in critically ill patients for various drugs in this class [32-34]. As a result, β-lactam TDM is becoming an increasingly valued part of clinical care of critically ill patients [21, 35-36].
In order to facilitate TDM, quantitative drug assays need to provide precise results with short turnaround time. For cefepime, drug concentrations are most often measured using validated high-performance liquid chromatography (HPLC) or liquid chromatography-tandem mass spectrometry (LC-MS/MS) methods [37-40]. Cefepime poses an added challenge for TDM due to rapid ex-vivo degradation in plasma at room temperature, requiring that all processing occur at 4°C [39]. The purpose of this review is to list the various LC-MS/MS methods in human blood, plasma, and serum for quantitation of cefepime with information on sample preparation, chromatographic and mass spectrometric conditions and validation parameters (Table 1) and to discuss relevant bioanalytical strategies and considerations for implementation of LC-MS/MS assays for therapeutic drug monitoring (TDM) of cefepime.
LC-MS/MS methods for cefepime analysis
Cefepime (Figure 1, CAS no. 88040-23-7, Pyrrolidinium,1-[[(6R,7R)-7-[[(2Z)-(2-amino-4-thiazolyl)(methoxyimino)acetyl]amino]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-en-3-yl]methyl]-1-methyl-, inner salt) has a chemical formula of C19H24N6O5S2 with an average molecular weight of 480.56 g/mol.
Sample preparation
Sample preparation is a critical step in bioanalytical method development. Due to limited stability of cefepime in plasma and serum at room temperature, samples should be immediately placed on ice after collection and processed at 4˚C temperature to provide accurate results. Plasma [41,42] and serum [43-45] samples have been used as the biological matrix for cefepime TDM assays.
Sample cleanup by removal of interfering matrix components (proteins, salts, and lipids) is often necessary to reduce the risk of matrix effects in LC-MS/MS procedures and to provide required selectivity, sensitivity, and ruggedness. The two most common methods for sample cleanup are (i) protein precipitation and (ii) solid-phase extraction (SPE) for cefepime TDM assays. For protein precipitation, the majority of the reported assays utilize methanol or acetonitrile as the precipitation and extraction solvents. SPE with Oasis HLB cartridges was utilized by Ohmori et al. [43], and an online SPE cleanup with Oasis HLB column was employed by Zander et al. [45]. These assays utilized stable labeled 2D3-cefepime or 13C2D3-cefepime as an internal standard, which provides several advantages in the accurate quantitation of drug levels in biological samples such as faster run time, improvement in intra-injection reproducibility, reduction of matrix effects and better sensitivity.
Chromatography and detection
Reverse phase chromatography was utilized for reported methods to separate cefepime from other molecules in the biological sample extract by partitioning between the mobile and stationary phases. Ammonium formate buffer or water with 0.1% formic acid was used as the aqueous mobile phase and methanol or acetonitrile with 0.1% formic acid was used as organic mobile phase. Nonpolar or hydrocarbon-like systems (C8, C18, Phenyl and F5) were used as the stationary phases. The majority of reported LC-MS/MS methods utilized tandem mass spectrometry for the detection and quantitation of cefepime, with the exception of Lefeuvre et al [41]. Cefepime is an inner salt and readily ionizes in the positive mode to provide excellent response. Multiple reaction monitoring transitions used by various methods is summarized in Table 1. The most common products of cefepime fragmentation that were utilized for quantitation were m/z 481.0 [M + H]+ → m/z 167 and 86.1 [42-44]. In some cases, doubly charged ion of cefepime m/z 241 [M + 2H]2+ was used to monitor the product m/z 227 for quantitation [41,45].
Figures and Tables
[Click to enlarge]
Discussion
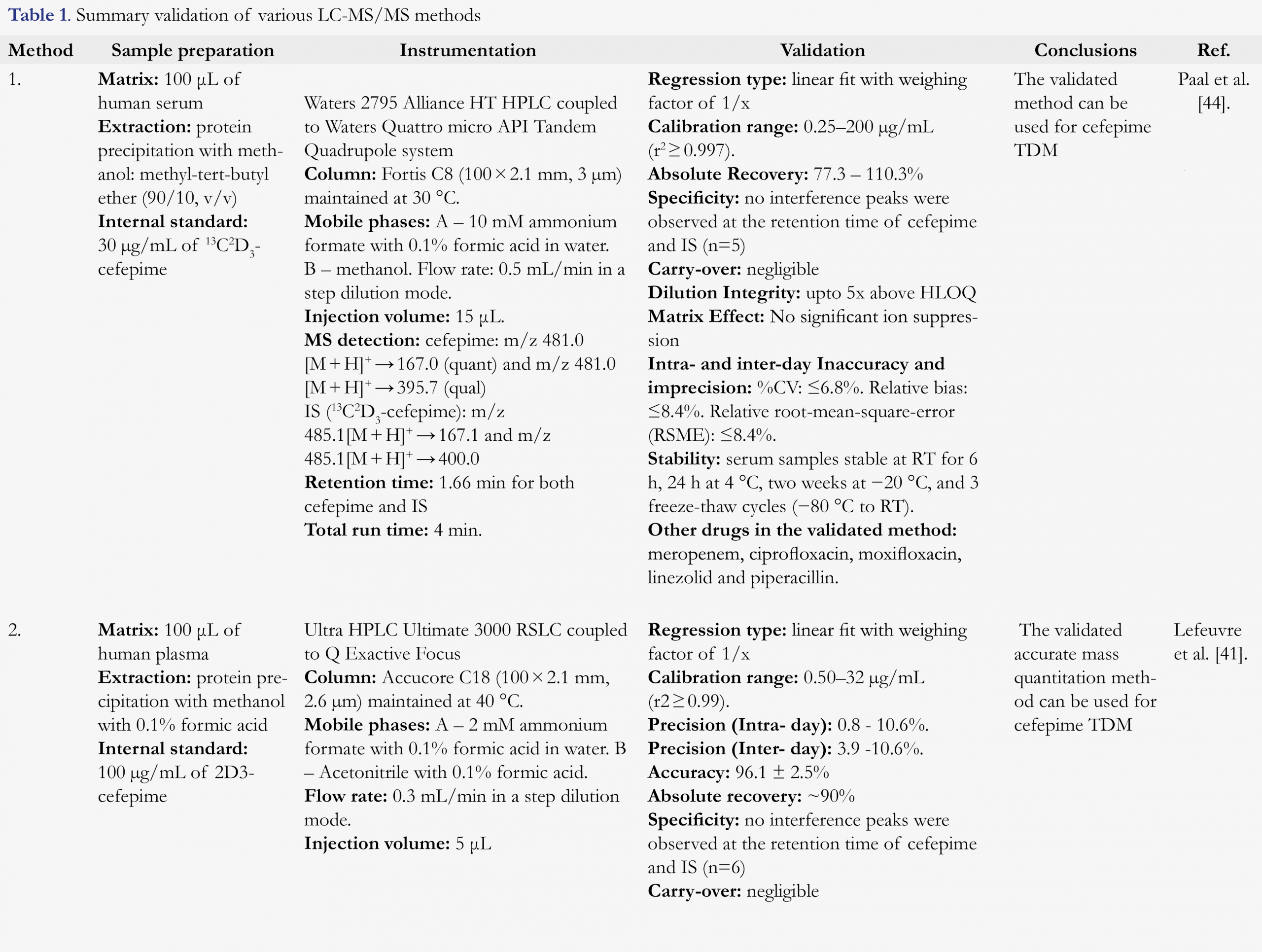
Recently published LC-MS/MS assays for cefepime TDM are summarized in Table 1. Paal et al. [44], reported a HPLC-MS/MS method for quantification of cefepime along with five other antibiotics in human serum (Table 1). The method employs a simple sample cleanup by protein precipitation and analysis utilizing stable labeled internal standards. Method utilized separation with C8 reverse phase HPLC column followed by MS/MS detection with clinically relevant concentration ranges. The assay demonstrated excellent selectivity, linearity and dilution integrity. Analytes were stable for 6 h at room temperature, 24 h at 4 °C, and two weeks at -20 °C.
Lefeuvre et al. [41], reported an UHPLC-HRMS assay for the simultaneous quantitation of cefepime and 14 other antibiotics in human plasma (Table 1). Sample cleanup involved protein precipitation followed by separation with C18 reverse phase chromatography. High-resolution full scan data acquisition was utilized for detection. Cefepime was stable for 48 h at room temperature and 2 – 8 °C and 20 days at -20 °C. This UHPLC-HRMS assay has the advantage of using acquired data for the retrospective analysis of metabolites.
Rigo-Bannin et al. [42], developed and validated a UHPLC-MS/MS method for simultaneous quantitation of ten β-lactam antibiotics in human plasma (Table 1). Sample cleanup involved protein precipitation, dilution, C18 reverse phase chromatographic separation followed by tandem mass spectrometric detection. The method demonstrated good linearity, selectivity and robustness to be utilized for TDM analysis. Cefepime was stable for 3 days at room temperature and 5 ± 3 °C and 6 months at -75 ± 3 °C.
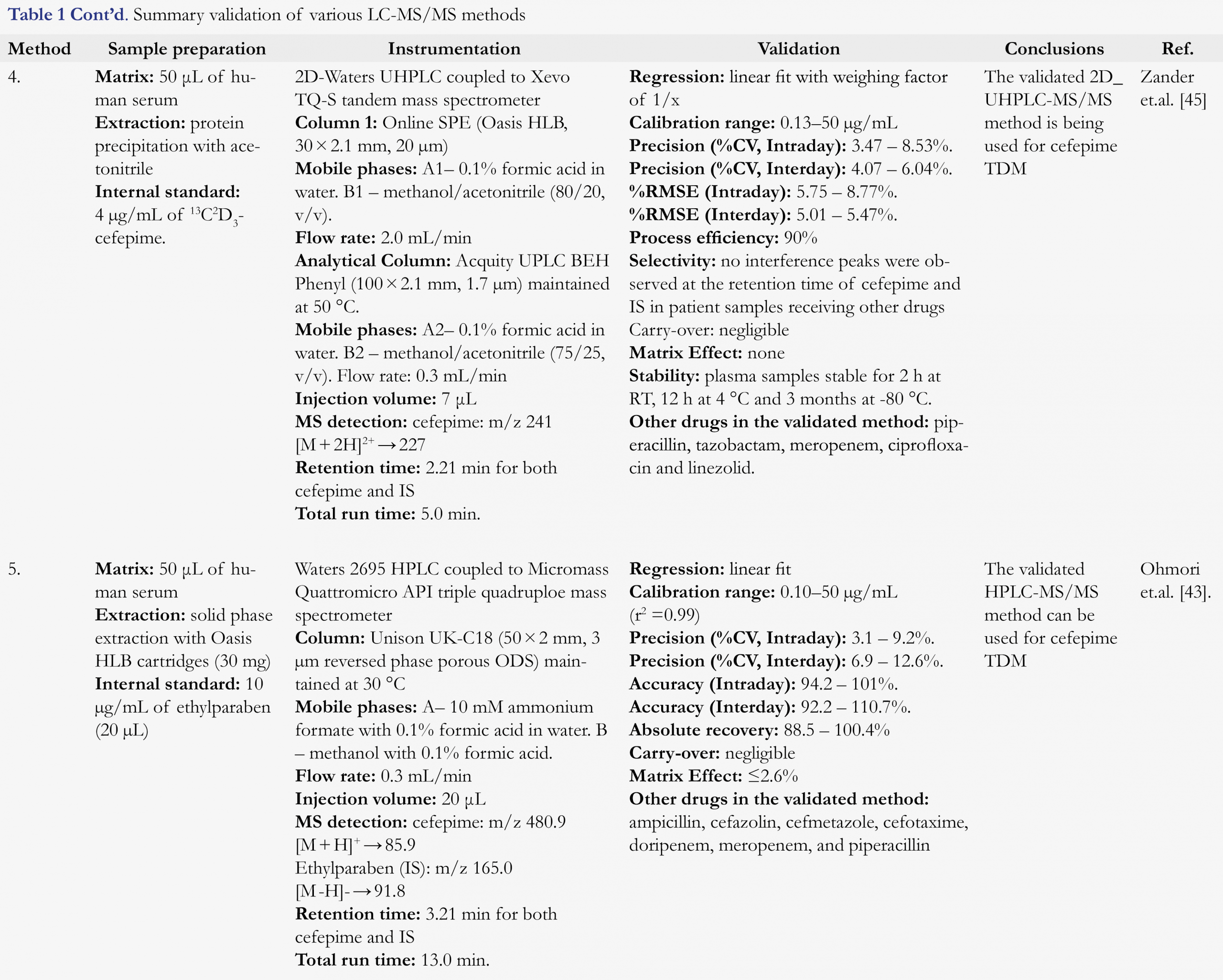
Zander et al. [45], reported a UHPLC-MS/MS method for analysis of eight β-lactam antibiotics in human serum (Table 1). Sample cleanup involved SPE followed by C18 reverse phase chromatographic separation and tandem mass spectrometric detection. The assay demonstrated to be robust and successfully employed for the analysis of clinical samples.
Methods reported by Paal et al. [44], and Rigo-Bannin et al. [42], involved simple sample cleanup, robust chromatographic separation and tandem mass spectrometric detection. While other methods used complex sample preparation, 2D-HPLC or HRMS detection. Method by Paal et al. [44] is robust for analysis of cefepime in human serum with an efficient sample preparation and LC-MS/MS analysis. While method by Rigo-Bannin et al. [42] is robust for analysis of cefepime in human plasma with simple sample cleanup and UPLC-MS/MS analysis.
Cefepime has limited stability in human plasma and serum at room temperature and the duration of acceptable stability varies from 3 h to 3 days at room temperature based on different reports (Table 1). However, the reason for these significant differences in the stability of cefepime in human plasma and serum at room temperature remains unresolved. It is critical to establish sufficient stability of cefepime during sample collection, storage, and processing to generate accurate quantitavie bioanalytical results.
Clinical Applications and Future Directions
In the current era of increasing antimicrobial resistance, it is paramount that antimicrobial dosing is personalized to assure optimal exposures that will result in clinical cure while minimizing the selection of resistant organisms. Therapeutic drug monitoring allows clinicians to provide individualized, target-oriented dosing for β-lactam antibiotics, including cefepime. Empiric dosing strategies, based on population PK modeling and Monte Carlo simulations, are designed to maximize the probability of target attainment. However, inter- and intra-variability in drug clearance and the volume of distribution prevents the use of a one-size-fits all dosing approach. The availability of LC-MS/MS assays that provide rapid and accurate measurement of cefepime concentrations can aid clinical pharmacists and clinicians in efforts to confirm drug exposures in individual patients, as well as facilitate adjustment of dosing in real-time to achieve desired levels. This is especially important in critically ill patients who require precise drug exposures to maximize bacterial killing and minimize the risk of toxicity. For instance, in a patient with a documented Gram-negative infection, clinicians can utilize TDM to estimate fT>MIC utilizing a variety of methods (i.e. Bayesian dose adaptation, log-linear regression, etc.). These data can then inform dose adjustments that aim to maximize the probability of target attainment in that patient. As described above, a number of PD targets have been associated with improved effectiveness outcomes, with >60% fT>MIC being the minimum time-dependent goal. TDM also holds potential to provide exposures that limit the selection of antimicrobial resistant phenotypes, although this has been predominantly established in vitro [14].
TDM in infants and children is challenged by a desire to minimize blood sampling, restrict entry into vascular access devices (i.e. central venous catheters), and avoid painful procedures such as venipuncture. Microsampling techniques can improve the performance of pediatric PK studies [46-48], applying less invasive approaches to blood sampling and reducing the risks to the patient. The availability of validated cefepime assays that utilize as little as 50 µL of plasma or serum can facilitate the clinical application of microsampling techniques and promote TDM in patients where sampling is challenging. Furthermore, application of whole blood microsampling (10-20 µL) to quantify cefepime levels in pediatric patints will be a convenient and efficient approach for TDM. Recently, Barco et al. [49], developed and validated a volumetric absorptive microsampling (VAMS™) assay for four antibiotics in human whole blood. VAMS™ approach allowed for accurate quantitation of drugs with no significant influence of hematocrit values. However, to employ VAMS™ approach for clinical studies, optimal collection, drying, shipping, and storage conditions with minimal drug degradation needs to be established. In addition, utility of VAMS™ approaches for TDM require converting whole blood concentrations to plasma or serum concentrations, where previously established reference ranges are available. Further studies comparing VAMS™, venous blood, and plasma samples are required to assess any potential difference between capillary blood from finger pricks and venous blood.
Acknowledgements
This work was supported through cooperative agreements from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services with the sites of the Collaborative Pediatric Critical Research Network. KJD is supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number K23HD091365.
Abbreviations
ACN, acetonitrile; APCI, atmospheric pressure chemical ionization; CV, coefficient of variation; ESI, electrospray ionization; IS, internal standard; F/T, freeze–thaw; HLB, hydrophilic–lipophilic based; HRMS, high resolution mass sepectrometry; IS, internal standard; LC, liquid chromatography; MeOH, methanol; MRM, multiple reaction monitoring; ODS, octadecylsilyl; PK, pharmacokinetic(s); RSD, relative standard deviation; SPE, solid‐phase extraction; TBME, ter‐butyl methyl ether; UHPLC, ultra‐high performance liquid‐chromatography; UPLC, ultra‐performance liquid‐chromatography.
References
1. Barradell LB, Bryson HM. Cefepime. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use, Drugs 47, 471-505 (1994). [CrossRef]
2. Tamma PD, Girdwood SC, Gopaul R, Tekle T, Roberts AA, Harris AD, Cosgrove SE, Carroll KC. The use of cefepime for treating AmpC β-lactamase-producing Enterobacteriaceae, Clin Infect Dis 57, 781-788 (2013). [CrossRef]
3. Akhabue A, Synnestvedt M, Weiner MG, Bilker WB, Lautenbach E. Cefepime-resistant Pseudomonas aeruginosa. Emerg Infect Dis 17, 1037-1043 (2011). [Crossref]
4. Barbhaiya RH, Forgue ST, Gleason CR, Knupp CA, Pittman KA, Weidler DJ, Movahhed H, Tenney J, Martin RR. Pharmacokinetics of cefepime after single and multiple intravenous administrations in healthy subjects. Antimicrob Agents Chemother 36, 552-557 (1992). [CrossRef]
5. Van der Auwera P, Santella PJ. Pharmacokinetics of cefepime: a review. J Antimicrob Chemother 32 (Suppl B), 103-115 (1993). [CrossRef]
6. Reed MD, Yamashita TS, Knupp CK, Veazey JM, Blumer JL. Pharmacokinetics of intravenously and intramuscularly administered cefepime in infants and children, Antimicrob Agents Chemother 41, 1783-1787 (1997). [CrossRef]
7. Capparelli E, Hochwald C, Rasmussen M, Parham A, Bradley J, Moya F. Population pharmacokinetics of cefepime in the neonate. Antimicrob Agents Chemother 49, 2760-2766 (2005).
8. Malone RS, Fish DN, Abraham E, Teitelbaum I. Pharmacokinetics of cefepime during continuous renal replacement therapy in critically ill patients. Antimicrob Agents Chemother 45, 3148-3155 (2001). [CrossRef]
9. Tam VH, McKinnon PS, Akins RL, Drusano GL, Rybak MJ. Pharmacokinetics and pharmacodynamics of cefepime in patients with various degrees of renal function. Antimicrob Agents Chemother 47, 1853-1861 (2003). [CrossRef]
10. Crandon JL, Bulik CC, Kuti JL, Nicolau DP. Clinical pharmacodynamics of cefepime in patients infected with Pseudomonas aeruginosa. Antimicrob Agents Chemother 54, 1111-1116 (2010). [CrossRef]
11. McKinnon PS, Paladino JA, Schentag JJ. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents 31, 345-351 (2008). [CrossRef]
12. Roberts JA, Paul SK, Akova M et al. DALI: defining antibiotic levels in intensive care unit patients: are current beta-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis 58, 1072-1083 (2014). [CrossRef]
13. Aitken SL, Altshuler J, Guervil DJ, Hirsch EB, Ostrosky-Zeichner LL, Ericsson CD, Tam VH. Cefepime free minimum concentration to minimum inhibitory concentration (fCmin/MIC) ratio predicts clinical failure in patients with Gram-negative bacterial pneumonia. Int J Antimicrob Agents 45, 541-544 (2015). [CrossRef]
14. Tam VH, Chang KT, Zhou J, et al. Determining beta-lactam exposure threshold to suppress resistance development in Gram-negative bacteria. J Antimicrob Chemother 72, 1421-1428 (2017). [CrossRef]
15. Craig WA, Ebert SC. Killing and regrowth of bacteria in vitro: a review, Scand J Infect Dis Suppl 74, 63-70. (1990)
16. Bhat SV, Peleg AY, Lodise TP, Shutt KA, Capitano B, Potoski BA, Paterson DL. Failure of current cefepime breakpoints to predict clinical outcomes of bacteremia caused by gram-negative organisms. Antimicrob Agents Chemother 51, 4390-4395 (2007). [CrossRef]
17. CLSI, Performance Standards for Antimicrobial Susceptibility Testing., Twenty-Fourth Informational Supplement M100. CLSI document M100-S24; Wayne, PA: Clinical and Laboratory Standards Institute (2014).
18. Taccone FS, Laterre PF, Dugernier T et al. Insufficient beta-lactam concentrations in the early phase of severe sepsis and septic shock. Crit Care 14, R126 (2010). [CrossRef]
19. Huttner A, Von Dach E, Renzoni A et al. Augmented renal clearance, low beta-lactam concentrations and clinical outcomes in the critically ill: an observational prospective cohort study. Int J Antimicrob Agents 45, 385-392 (2015). [CrossRef]
20. Shoji K, Bradley JS, Reed MD, van den Anker JN, Domonoske C, Capparelli EV. Population Pharmacokinetic Assessment and Pharmacodynamic Implications of Pediatric Cefepime Dosing for Susceptible-Dose-Dependent Organisms. Antimicrob Agents Chemother 60, 2150-2156 (2016). [CrossRef]
21. Charmillon A, Novy E, Agrinier N et al. The ANTIBIOPERF study: a nationwide cross-sectional survey about practices for β-lactam administration and therapeutic drug monitoring among critically ill patients in France. Clin Microbiol Infect 22, 625-631 (2016). [CrossRef]
22. Cheatham SC, Shea KM, Healy DP et al. Steady-state pharmacokinetics and pharmacodynamics of cefepime administered by prolonged infusion in hospitalised patients. Int J Antimicrob Agents 37, 46-50 (2011). [CrossRef]
23. Yu Z, Pang X, Wu X, Shan C, Jiang S. Clinical outcomes of prolonged infusion (extended infusion or continuous infusion) versus intermittent bolus of meropenem in severe infection: A meta-analysis. PloS one 13, e0201667 (2018). [CrossRef]
24. Lee YR, Miller PD, Alzghari SK, Blanco DD, Hager JD, Kuntz KS. Continuous Infusion Versus Intermittent Bolus of Beta-Lactams in Critically Ill Patients with Respiratory Infections: A Systematic Review and Meta-analysis. Eur J Drug Metab Pharmacokinet 43, 155-170 (2018). [CrossRef]
25. Bradford PA. Extended-spectrum beta-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev 14, 933-951 (2001). [CrossRef]
26. Canton R, Gonzalez-Alba JM, Galan JC. CTX-M Enzymes: Origin and Diffusion. Front Microbiol 3, 110 (2012). [CrossRef]
27. Park YS, Adams-Haduch JM, Shutt KA et al. Clinical and microbiologic characteristics of cephalosporin-resistant Escherichia coli at three centers in the United States. Antimicrob Agents Chemother 56, 1870-1876 (2012).
28. Beumier M, Casu GS, Hites M et al. Elevated beta-lactam concentrations associated with neurological deterioration in ICU septic patients. Minerva Anestesiol 81, 497-506 (2015).
29. Huwyler T, Lenggenhager L, Abbas M et al. Cefepime plasma concentrations and clinical toxicity: a retrospective cohort study. Clin Microbiol Infect 23, 454-459 (2017). [CrossRef]
30. Lamoth F, Alexander BD. Nonmolecular methods for the diagnosis of respiratory fungal infections. Clin Lab Med 34, 315-336 (2014). [CrossRef]
31. Durand-Maugard C, Lemaire-Hurtel AS, Gras-Champel V et al. Blood and CSF monitoring of cefepime-induced neurotoxicity: nine case reports. J Antimicrob Chemother 67, 1297-1299 (2012). [CrossRef]
32. De Waele JJ, Carrette S, Carlier M et al. Therapeutic drug monitoring-based dose optimisation of piperacillin and meropenem: a randomised controlled trial. Intensive Care Med 40, 380-387 (2014). [CrossRef]
33. Sime FB, Roberts MS, Tiong IS et al. Can therapeutic drug monitoring optimize exposure to piperacillin in febrile neutropenic patients with haematological malignancies? A randomized controlled trial. J Antimicrob Chemother 70, 2369-2375 (2015). [CrossRef]
34. Heil EL, Nicolau DP, Farkas A, Roberts JA, Thom KA. Pharmacodynamic target attainment for cefepime, meropenem and piperacillin/tazobactam using a pharmacokinetic/pharmacodynamic-based dosing calculator in critically ill patients. Antimicrob Agents Chemother 62(9), e01008-18 (2018). [CrossRef]
35. Wong G, Brinkman A, Benefield RJ et al. An international, multicentre survey of beta-lactam antibiotic therapeutic drug monitoring practice in intensive care units. J Antimicrob Chemother 69, 1416-1423 (2014). [CrossRef]
36. Sime FB, Roberts MS, Peake SL, Lipman J, Roberts JA. Does Beta-lactam Pharmacokinetic Variability in Critically Ill Patients Justify Therapeutic Drug Monitoring? A Systematic Review. Ann Intensive Care 2, 35 (2012). [CrossRef]
37. El-Najjar N, Hösl J, Holzmann T, Jantsch J, Gessner A. UPLC-MS/MS method for therapeutic drug monitoring of 10 antibiotics used in intensive care units. Drug Test Anal 10, 584-591 (2018). [CrossRef]
38. Carlier M, Stove V, De Waele JJ, Verstraete AG. Ultrafast quantification of β-lactam antibiotics in human plasma using UPLC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci 978-979, 89-94 (2015). [CrossRef]
39. Bugnon D, Giannoni E, Majcherczyk P, Glauser MP, Moreillon P. Pitfalls in cefepime titration from human plasma: plasma-and temperature-related drug degradation in vitro. Antimicrob Agents Chemother 46, 3654-3656 (2002). [CrossRef]
40. D’Cunha R, Bach T, Young BA et al. Quantification of Cefepime, Meropenem, Piperacillin and Tazobactam in Human Plasma using a Sensitive and Robust LC-MS/MS Method- Part I. Assay Development and Validation. Antimicrob Agents Chemother 62(9), e00861-18 (2018).
41. Lefeuvre S, Bois-Maublanc J, Hocqueloux L et al. A simple ultra-high-performance liquid chromatography-high resolution mass spectrometry assay for the simultaneous quantification of 15 antibiotics in plasma. J Chromatogr B Analyt Technol Biomed Life Sci 1065-1066, 50-58 (2017). [CrossRef]
42. R. Rigo-Bonnin, A. Ribera, A. Arbiol-Roca et al. Development and validation of a measurement procedure based on ultra-high performance liquid chromatography-tandem mass spectrometry for simultaneous measurement of beta-lactam antibiotic concentration in human plasma. Clin Chim Acta 468, 215-224 (2017). [CrossRef]
43. Ohmori T, Suzuki A, Niwa T et al. Simultaneous determination of eight beta-lactam antibiotics in human serum by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 879, 1038-1042 (2011). [CrossRef]
44. Paal M, Zoller M, Schuster C, Vogeser M, Schutze G. Simultaneous quantification of cefepime, meropenem, ciprofloxacin, moxifloxacin, linezolid and piperacillin in human serum using an isotope-dilution HPLC-MS/MS method. J Pharm Biomed Anal 152, 102-110 (2018). [CrossRef]
45. Zander J, Maier B, Suhr A et al. Quantification of piperacillin, tazobactam, cefepime, meropenem, ciprofloxacin and linezolid in serum using an isotope dilution UHPLC-MS/MS method with semi-automated sample preparation. Clin Chem Lab Med 53, 781-791 (2015). [CrossRef]
46. Guerra Valero YC, Wallis SC, Lipman J, Stove C, Roberts JA, Parker SL. Clinical application of microsampling versus conventional sampling techniques in the quantitative bioanalysis of antibiotics: a systematic review. Bioanalysis, 10, 407-423 (2018). [CrossRef]
47. Parker SL, Dorofaeff T, Lipman J et al. Is there a role for microsampling in antibiotic pharmacokinetic studies? Expert Opin Drug Metab Toxicol 12, 601-614 (2016). [CrossRef]
48. Dorofaeff T, Bandini RM, Lipman J, Ballot DE, Roberts JA, Parker SL. Uncertainty in Antibiotic Dosing in Critically Ill Neonate and Pediatric Patients: Can Microsampling Provide the Answers? Clin Ther 38, 1961-1975 (2016). [CrossRef]
49. Barco S, Castagnola E, Moscatelli A, Rudge J, Tripodi G, Cangemi G. Volumetric adsorptive microsampling-liquid chromatography tandem mass spectrometry assay for the simultaneous quantification of four antibiotics in human blood: Method development, validation and comparison with dried blood spot. J Pharm Biomed Anal 145, 704-710 (2017). [CrossRef]
All site content, except where otherwise noted, is licensed under a Creative Commons Attribution 4.0 License