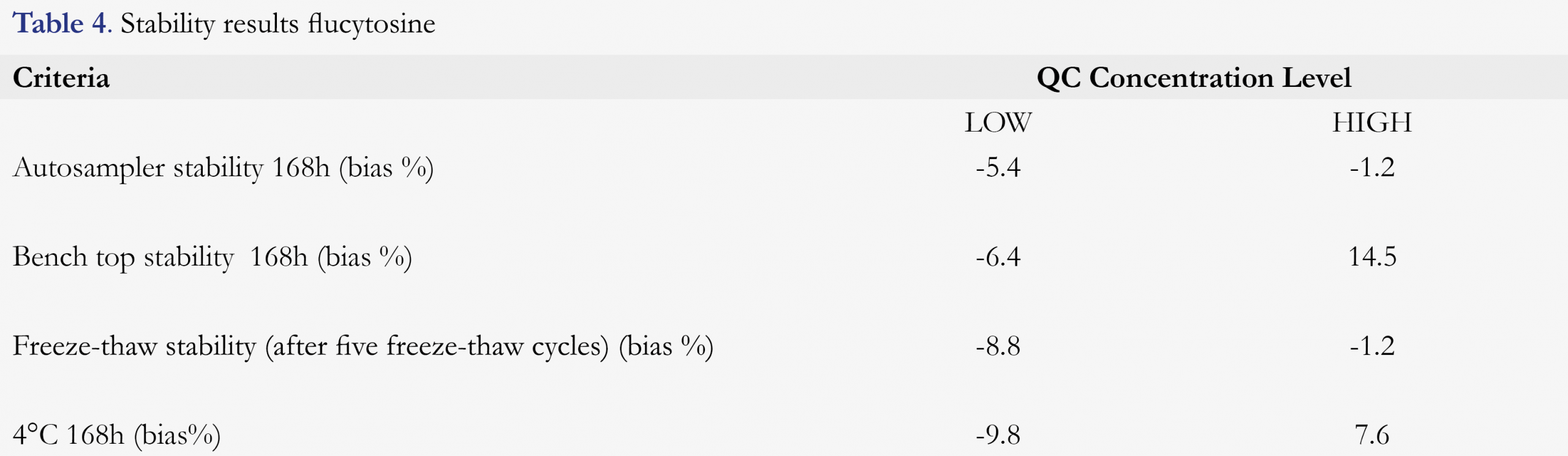OPEN-ACCESS PEER-REVIEWED
RESEARCH ARTICLE
Jan-Willem Alffenaar1,*, Kai van Hateren1, Daan Touw1,2
1University of Groningen, University Medical Center Groningen, Department of Clinical Pharmacy and Pharmacology, Groningen, The Netherlands. 2University of Groningen, Groningen Research Institute of Pharmacy, Unit Pharmacokinetics, Toxicology and Targeting, Groningen, The Netherlands.
Journal of Applied Bioanalysis. Vol.4. No.5. pages 157-165 (2018)
Published 15 December 2018. https://doi.org/10.17145/jab.18.020 | (ISSN 2405-710X)
*Correspondence:
Alffenaar JW . University Medical Center Groningen, Hanzeplein 1, 9713 GZ Groningen
the Netherlands, Phone: +31 503614070, Fax +31 503614087.
Citation:
Alffenaar JW, van Hateren K, Touw DJ. Determination of Flucytosine in Human Serum using Liquid Chromatography-Tandem Mass Spectrometry. J Appl Bioanal 4(5), 157-165 (2018).
Editor: Dr. Sophie Acyriex, Université Claude Bernard Lyon 1, Villeurbanne, France.
Open-access and Copyright:
©2018 Alffenaar JW et al. This article is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Funding/Manuscript writing assistance:
The authors have no financial support or funding to report and they also declare that no writing assistance was utilized in the production of this article.
Competing interest:
The authors have declared that no competing interest exists.
Article history:
Received 18 October 2018, Revised 25 November 2018, Accepted 28 November 2018.
Abstract
Objectives
Flucytosine is an important drug for the combination treatment of cryptococcal meningitis in patients suffering from an infection with human immunodeficiency virus. As new synergistic regimens are being explored in clinical trials a full oral regimen may be near. For such a study evaluation of drug exposure is of critical importance as bioavailability could be compromised and elimination highly depends on renal function. No simple LC-MS/MS method for therapeutic drug monitoring of flucytosine has been published.
Methods
Therefore, a new method using a triple quadrupole mass spectrometer was developed for analysis of flucytosine in human serum, using stable isotopically labeled flucytosine (2H15N-flucytosine) as internal standard.
Results
The calibration curve was linear over a range of 2.00 (LLOQ) to 100.0 mg/L with a correlation coefficient of 0.9993. The calculated accuracy ranged from -11.1 % to -1.4 %. Within-run precision ranged from 1.6 % to 4.3 % and between-run precision ranged from 0.0 % to 5.4 %. Evaluation of serum concentrations during routine TDM showed a large variability in serum concentrations. The calibration curve was linear over a range of 2.00 (LLOQ) to 100.0 mg/L with a correlation coefficient of 0.9993. The calculated accuracy ranged from -11.1 % to -1.4 %. Within-run precision ranged from 1.6 % to 4.3 % and between-run precision ranged from 0.0 % to 5.4 %. Evaluation of serum concentrations during routine TDM showed a large variability in serum concentrations.
Conclusions
We conclude that a simple LC-MS/MS method to quantify flucytosine in human serum has successfully been validated and this assay can be used for routine therapeutic drug monitoring as well as clinical studies.
Keywords
Flucytosine, mass spectrometry, chromatography, therapeutic drug monitoring, crytococcus meningitis.
Introduction
Flucytosine is a fluorinated pyrimidine analogue with antifungal activity that has been available for more than 70 years. Flucytosine is nowadays mostly used for the treatment of Cryptococcus infections in human immunodeficiency virus infected patients [1]. Flucytosine should not be used alone due to its relative weak activity as monotherapy and its rapidly acquired drug resistance [2]. Therapy with amphotericin B and fluconazole has been considered to be standard of care because the outcome of this regimen has been favourable in most of the patients if diagnosed early [2].
As Cryptococcus infection is a poverty related disease newly developed drugs are scarce. Very promising are the results of the repurposed drug sertraline which showed a faster clearance of the cerebrospinal fluid when added to standard therapy [3]. Oral therapy is preferred over intravenously (IV) administered therapy in resource limited settings especially when the diagnosis can be made early [1]. However, pharmacokinetic variability may be higher compared to IV therapy due to malabsorption. Understanding drug exposure in relation to treatment outcome is therefore important [4]. The pharmacodynamics of the old drug flucytosine in combination with amphotericin B was addressed just recently after being on the market for decades. The authors concluded that alternative dosing strategies may be equally effective but less toxic [5].
Since the antimicrobial action of flucytosine is exposure dependent, it is critical that target exposures is achieved. The major reasons for TDM of flucytosine are altered bioavailability and altered renal function potentially resulting in either subtherapeutic or toxic flucytosine serum concentrations. Although dosing guidelines provide general recommendations how to adjust the dose in case of renal function loss, these may not result in adequate drug concentrations in all patients. To tailor the dose to reduce toxicity and improve efficacy therapeutic drug monitoring (TDM) has been recommended [6–8].
To enable TDM a bioanalytical method is required to measure drug concentrations. Several methods have been described using high-performance liquid chromatography (HPLC) with ultraviolet-visible (UV-VIS) detection [9–11]. However, to be able to guide flucytosine therapy the turnaround time should be as fast as possible and laboratory delay should be avoided. The HPLC-UV-VIS methods generally are time consuming due to necessary sample clean-up and therefore liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) can be preferred over conventional HPLC methods due to its simple sample treatment and fast chromatography [12].
Only two analytical methods using LC-MS/MS have been presented. The multi-analyte method by Toussaint et al. showed a substantial matrix effect. Although this was corrected by the use of a deuterated internal standard the chromatogram still showed asymmetric peaks [16].
The procedure described by Qu et al. used an advanced set up required to measure both amphotericin B and flucytosine resulting in a low linear range for flucytosine [14]. Due to the change to lipid formulations of amphotericin B, TDM of this drug is less necessary and this advanced assay seems less suitable for routine monitoring of serum concentrations of flucytosine. Therefore the aim of this study was to develop a simple LC-MS/MS method using a stable isotope of flucytosine as internal standard to quantify flucytosine in human serum.
Materials and methods
Assay development
Flucytosine and 2H15N-flucytosine (internal standard; Figure 1) were obtained from EDQM (Strasbourg, France) and Alsachim (Illkirch Graffenstaden, France), respectively. Water and ammonium formate were all of analytical grade suitable for LC-MS/MS. Human serum was made available according to local hospital guidelines.
For the preparation of calibration standards and Quality Control (QC) samples two flucytosine stock solutions of 4000 mg/L in 100% methanol were prepared and added to blank human serum. The amounts of stock solution added to the serum did not exceed 5% V/V of the total volume. On day 1 of the validation, calibration standards and QC samples were freshly prepared and stored at -20°C to be used at the other validation days. Concentrations of the standards were 2.00, 4.00, 10.0, 20.0, 40.0, 60.0, 80.0 and 100.0 mg/L. The concentrations of the QC samples are listed in Table 1. The internal standard solution was prepared by diluting a 1000 mg/L 2H15N-flucytosine stock solution to 1 mg/L with 100% methanol. Sample preparation included addition of 500 µL precipitation reagent to 10 µL serum, followed by 1 min of vortexing. The vials were centrifuged for 5 min at 11000 rpm followed by the injection into the LC-MS/MS system of 0.1 µL of the clear upper layer.
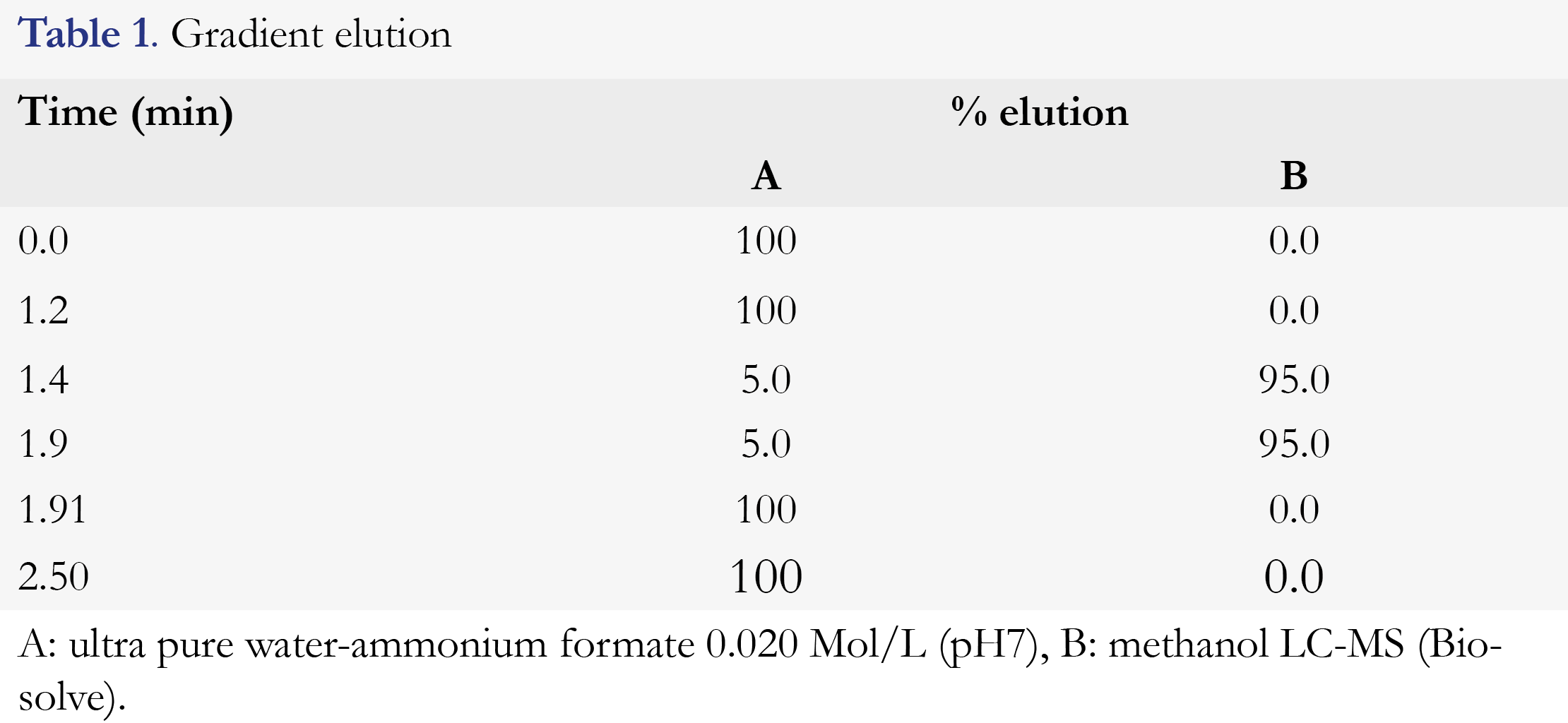
The analysis was performed on a 6460 triple quadrupole LC-MS/MS (Agilent, Santa Clara, CA, USA) with a 1290 Infinity binary pump VL and 1290 Infinity Autosampler (Santa Clara, CA, USA). The temperature of the autosampler was set at 10°C. A Waters Atlantis T3, analytical column, 100 x 2.1 mm, 3-μm (Waters Corporation, Milford, MA, USA) was used for chromatographic separation. Column temperature was set at 40°C. A flow of 500 μL/ min of the mobile phase was applied. The mobile phase consisted of an aqueous buffer (containing ammonium formate 0.02 mol/L, pH 7) and methanol. The elution gradient is shown in Table 1. The method had a runtime of 2.5 minutes.
The MS was configured onto positive electrospray ionization mode (Jet stream mode) and Selected Reaction Monitoring (SRM) with a capillary voltage of 1800 V, a gas temperature (sheats gas heater) of 200°C and a sheath gas pressure and auxiliary pressure of 12 and 18 arbitrary units respectively. Mass transitions for flucytosine were 130.1 m/z → 113.1 m/z and for 2H15N-flucytosine 132.1 m/z→114.2 m/z, using a scan width of 0.7 m/z. Collision energy was determined on 20 eV for both transitions. MassHunter software was applied for peak height integration for all components.
Analytical Method validation
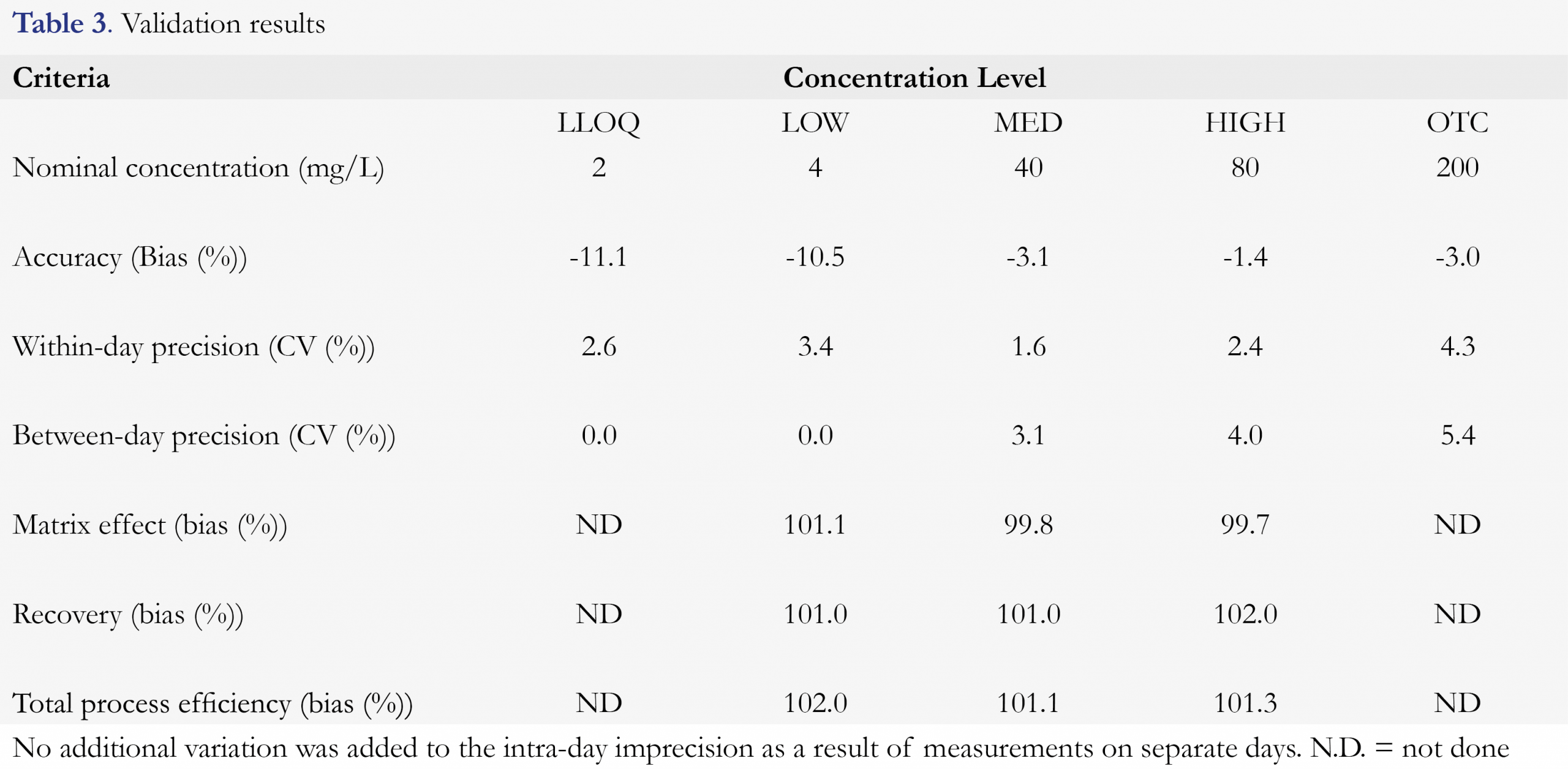
Validation of the method included selectivity and sensitivity, linearity, accuracy and precision, recovery, matrix effect, process efficiency and dilution integrity. The calibration curve of flucytosine consisted of 8 samples with concentrations of 2.00, 4.00, 10.0, 20.0, 40.0, 60.0, 80.0 and 100.0 mg/L. Quality Control (QC) samples with 4 different concentrations of flucytosine were used. The LLOQ was 2.00 mg/L, LOW was 4.00 mg/L, MED was 40.0 mg/L, HIGH was 80.0 mg/L and OTC was 200mg/L. The effect of endogenous substances was evaluated by testing 6 lots of different blank serum and subsequently compared with response of the LLOQ. During three days, on each day a single calibration curve was analyzed in quintuplicate per QC sample on three consecutive days. Evaluation of precision included within-run and between-run precision using the same method as accuracy. The accuracy and precision concentration bias and CV were calculated per run and were not allowed to exceed 20% for LLOQ and not to exceed 15% for other QC levels. One-way ANOVA was used to calculate within-run, between-run and overall CV’s. Procedures to evaluate recovery, matrix effect, process efficiency were described earlier by Matuszewski and colleagues [15]. The recovery was calculated by dividing the peak height of the extract of the spiked matrix by the peak height of the spiked extract of a blank matrix. The matrix effect was tested by dividing the peak height of the spiked extract of the blank matrix by the peak height of the spiked extraction solution. The process efficiency was determined by dividing the peak height of the extract of the spiked matrix by the peak height of spiked extraction solution. Recovery, matrix effect and process efficiency were all determined at Low, Medium and High concentrations in quintuplicate in a single run. To determine the dilution integrity, on three consecutive days, a QC sample with flucytosine in a concentration of 200 mg/L in serum was diluted 10 times and subsequently measured in quintuplicate.
Stability of flucytosine was tested in different test conditions, including storage stability and freeze-thaw stability at low and high level QC. Stability of flucytosine was examined by evaluation of QC samples at room temperature (20°C -25°C), in a refrigerator at 4°C, and after sample preparation in the autosampler at 10°C. Stability was also tested using five freeze-thaw cycles at -20°C. Stability tests were done at LOW and HIGH concentration in quintuplicate per concentration. Stability was defined as a change in concentration and should be ≤ 15%. Long-term stability has been investigated earlier and flucytosine was proven to be stable for at least 30 days at -20°C and -80°C [13,14].
Clinical application and proficiency testing
Patients that are treated with flucytosine are routinely subjected to TDM in our hospital. According to the local standard operating procedure the target concentration for trough concentrations is 25-50 mg/L and 50-100 mg/L for peak concentrations. Toxic effects are expected at concentrations exceeding 100mg/L. We evaluated the use of our assay in routine TDM of flucytosine. Written informed consent of patients was not required because of the retrospective analysis of results from routine analyses. In addition to routine TDM the laboratory participated in an international proficiency testing program for antifungal drugs [16]. The performance of the analytical procedure is considered inaccurate if the reported results deviate >20% from the weighed-in concentration of the test sample.
Results
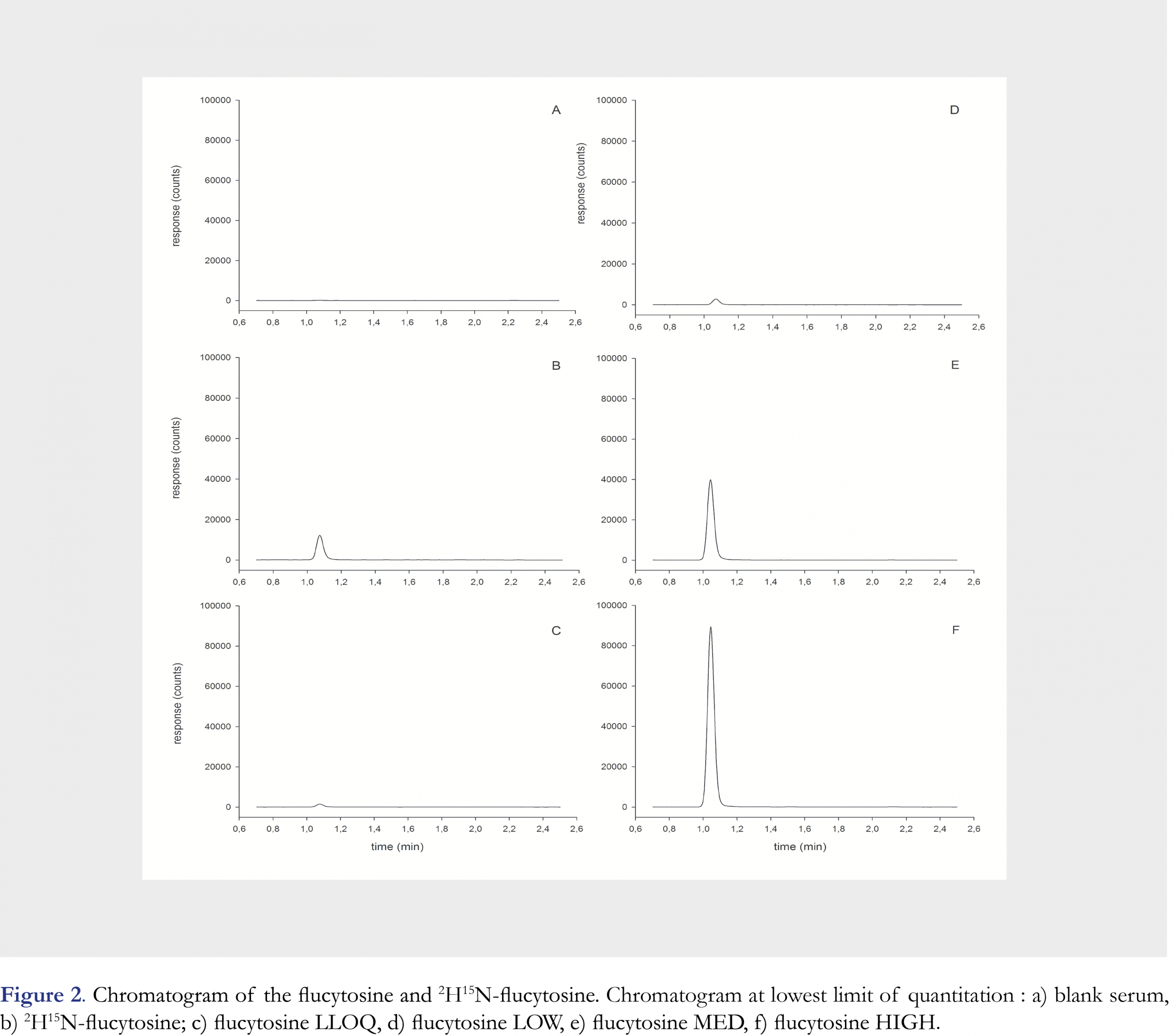
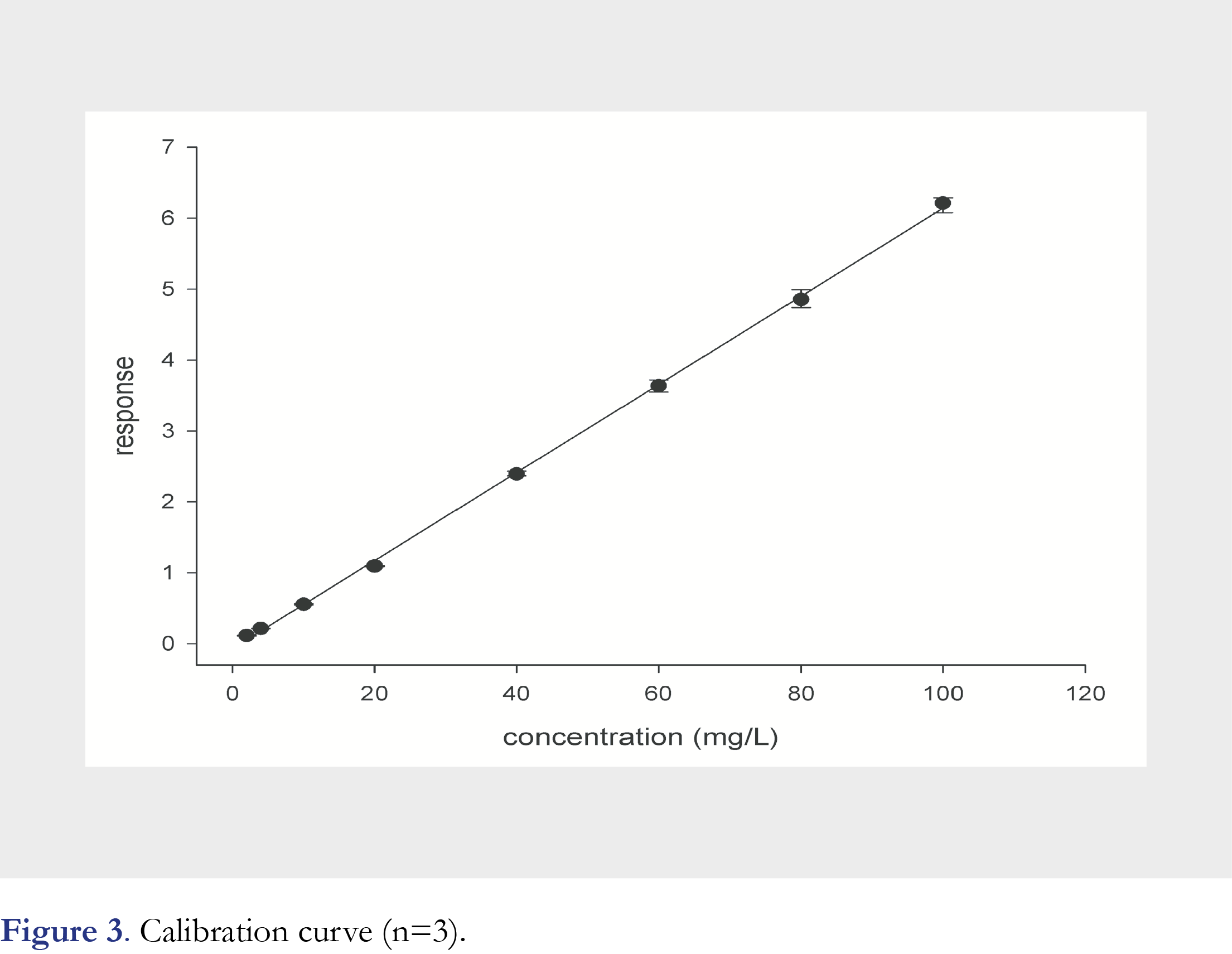
Mean retention times of flucytosine and 2H15N-flucytosine were equal to 1.01 minutes (Figure 2). Examining the selectivity of this analytical method, no interfering peaks at the retention time of flucytosine from endogenous substances were observed in any of the six different lots of human serum (Figure 2). The calibration curve was linear over a range of 2.0 to 100.0 mg/L and the correlation coefficient (R2) was 0.9993 (Figure 3). The calibration curve parameters are shown in Table 2.
The calculated accuracy ranged from -11.1% to -1.4% for flucytosine. Within-run precision ranged from 1.6 % to 4.3 % and between-run precision ranged from 0.0 % to 5.4%. The results of accuracy and precision for all QC levels for flucytosine are presented in Table 3 as well as recovery, matrix effect and process efficiency.
Stability of flucytosine at the different storage conditions is shown in Table 4. Measured concentrations of LOW and HIGH QC samples for the freeze-thaw stability biased between -8.8 % and -1.2 % for flucytosine. Stability was determined by measuring QC samples stored at 4°C and differed -9.8% and 7.6% from the nominal concentrations. After storage at room temperature for 168h, the concentrations biased from -6.4 % to 14.5 % for flucytosine compared to the initial concentrations. After sample preparation the concentrations of the samples stored in the autosampler for 168 h biased between -5.4 % and -1.2 % for flucytosine from the nominal concentrations.
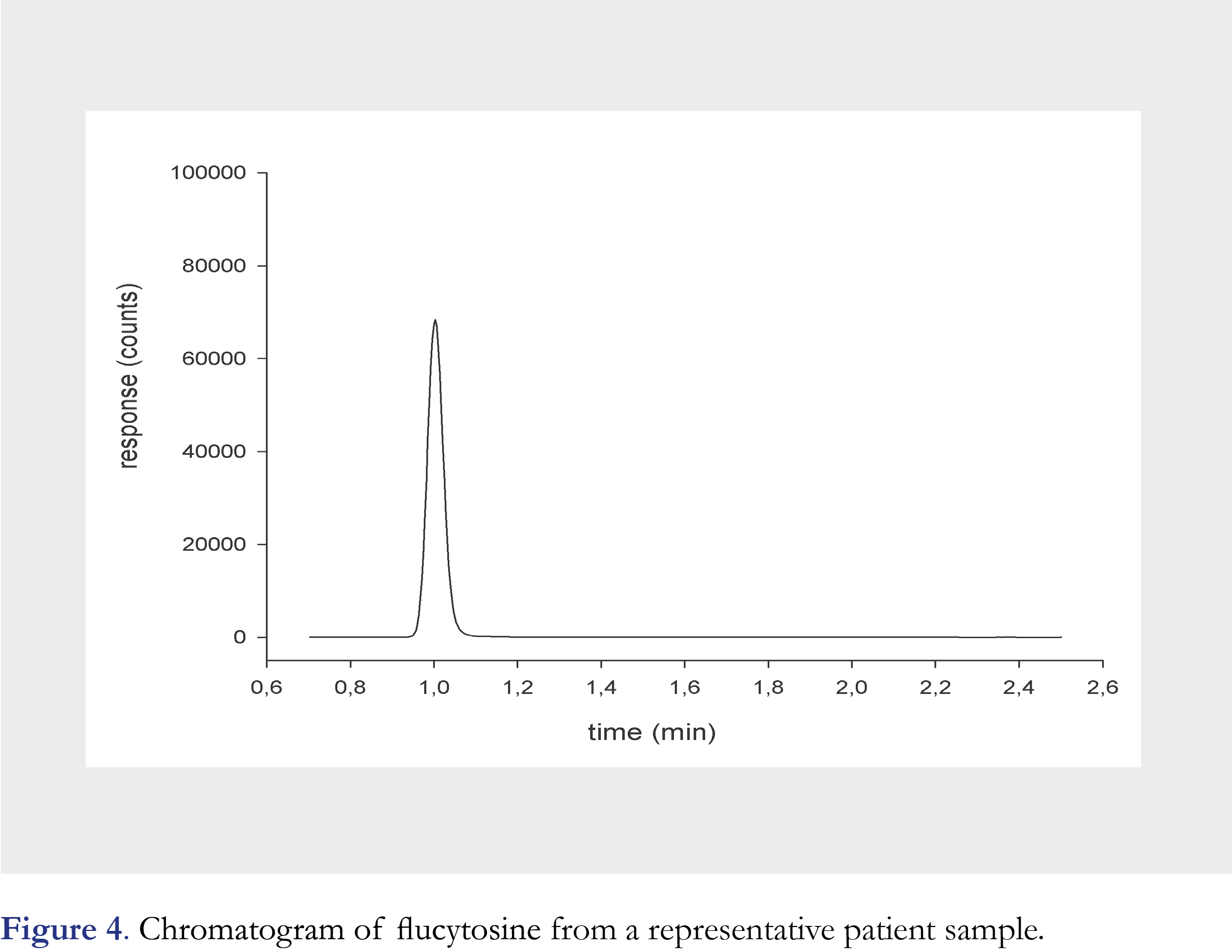
We report the results of 6 patients treated with flucytosine as part of their treatment and 29 samples were collected for TDM (Figure 4). The mean trough concentration was 40.4 (range 5.09-87) mg/L and mean peak concentration was 87.6 (range 45.5-128) mg/L. Two sets of test samples were received from the proficiency program since the validation of the method. The concentration of the test samples ranged from 16.2-92.0 mg/L. The reported result were accurate and ranged from 98.8-106% of the weighed-in concentration of the test samples.
Figures and Tables
[Click to enlarge]
Discussion
This study describes the validation of a simple method using a triple quadrupole LC-MS/MS method for the measurement of flucytosine in human serum and 2H15N-flucytosine as internal standard. This LC-MS/MS method showed good accuracy and precision. Linearity was shown for the range of 2.0 (LLOQ) – 100.0 mg/L.
Compared to the procedure described by Qu and coworkers the analytical range of our procedure had a higher HLQ which better suites daily TDM [14]. However, still a part of the peak samples had to be diluted. We recommend to routinely dilute the peak sample to avoid unnecessary delay if a peak concentration is found above the validated range.
During method development it became clear that the T3 column, specifically used for polar compounds, could be preferred over the standard columns as it considerably improved the retention. In addition the peak shape also improved. The peak shape was further improved by using an injection volume of 0.1 microliter which is within the specified range of the 1290 Infinity Autosampler. As shown by our validation reproducibility of the small injection volume is of no concern. The major advantage of the low injection volume is that it improves peak shape (Gaussian shape) and reduces matrix effect.
Simple protein precipitation as method for sample preparation is a major advantage of this LC-MS/MS method which makes it suitable for routine TDM. The run time is very short since the retention time of flucytosine is 1.01 minutes which was shorter than the multi-analyte assay by Toussaint [13]. This facilitates a high sample throughput. This is in line with the strategy of the World Health Organization, to increase capacity of high-quality laboratory systems using to support a clinical TDM service.
Lemper et al. presented the first results of an international proficiency testing program for measurement of flucytosine [16]. Participation in an interlaboratory proficiency testing program is a requirement for quality insurance and should be done on top of routine QC monitoring. This will ensure that serum drug concentrations are measured accurately which is a prerequisite for safe and adequate therapeutic drug monitoring. In addition, ISO regulations require to participate in external proficiency testing for laboratory accreditation and to make analytical results comparable between laboratories.
This method was developed for monitoring flucytosine serum concentrations for routine therapeutic drug monitoring but is also suitable for measuring drug concentrations in clinical trials or pharmacokinetic studies to explore the use of flucytosine in full oral anti-cryptococcus treatment regimens.
Conclusions
A simple LC-MS/MS method to measure flucytosine in human serum using a stable isotope as internal standard has been developed and validated. This method can be used in routine therapeutic drug monitoring.
References
1. Sloan D, Dlamini S, Paul N, Dedicoat M. Treatment of acute cryptococcal meningitis in HIV infected adults, with an emphasis on resource-limited settings. Cochrane Database Syst Rev 2008; CD005647. [CrossRef]
2. Zhou Y, Li G. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Chinese J Infect Chemother 10,161–166 (2010).
3. Rhein J, Morawski BM, Hullsiek KH, et al. Efficacy of adjunctive sertraline for the treatment of HIV-associated cryptococcal meningitis: An open-label dose-ranging study. Lancet Infect Dis 16, 809–818 (2016). [CrossRef]
4. Veringa A, van der Elst KCM, Day JN, Thwaites GE, Alffenaar JWC. Sertraline for HIV-associated cryptococcal meningitis. Lancet Infect Dis 16, 1111 (2016). [CrossRef]
5. O’Connor L, Livermore J, Sharp AD, et al. Pharmacodynamics of liposomal amphotericin b and flucytosine for cryptococcal meningoencephalitis: Safe and effective regimens for immunocompromised patients. J Infect Dis 208, 351–361 (2013). [CrossRef]
6. Pasqualotto AC, Howard SJ, Moore CB, Denning DW. Flucytosine therapeutic monitoring: 15 years experience from the UK. J Antimicrob Chemother 59, 791–793 (2007). [CrossRef]
7. Goodwin MI, Drew RH. Antifungal serum concentration monitoring: An update. J Antimicrob Chemother 61,17–25 (2008). [CrossRef]
8. Stott KE, Hope WW. Therapeutic drug monitoring for invasive mould infections and disease: Pharmacokinetic and pharmacodynamic considerations. J Antimicrob Chemother 72, i12-i18 (2017). [CrossRef]
9. Higashi T, Awada D, Shimada K. Simultaneous determination of flucytosine and fluorouracil in human plasma by high-performance liquid chromatography. Biomed Chromatogr 15, 89–94 (2001). [CrossRef]
10. Ng TK, Chan RC, Adeyemi-Doro FA, Cheung SW, Cheng AF. Rapid high performance liquid chromatographic assay for antifungal agents in human sera. J Antimicrob Chemother 37, 465–472 (1996). [CrossRef]
11. Serve KM, Yáñez JA, Remsberg CM, Davies NM, Black ME. Development and validation of a rapid and sensitive HPLC method for the quantification of 5-fluorocytosine and its metabolites. Biomed Chromatogr 24(5), 522-527 (2010).
12. Veringa A, Sturkenboom MGG, Dekkers BGJ, et al. LC-MS/MS for Therapeutic Drug Monitoring of anti-infective drugs. TrAC – Trends Anal Chem 84, 34-40 (2016). [CrossRef]
13. Fatiguso G, Favata F, Zedda I, et al. A simple high performance liquid chromatography–mass spectrometry method for Therapeutic Drug Monitoring of isavuconazole and four other antifungal drugs in human plasma samples. J Pharm Biomed Anal 145, 718–724 (2017). [CrossRef]
14. Qu L, Qian J, Ma P, Yin Z. Utilizing online-dual-SPE-LC with HRMS for the simultaneous quantification of amphotericin B, fluconazole, and fluorocytosine in human plasma and cerebrospinal fluid. Talanta 165, 449-457 (2017). [CrossRef]
15. Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem 75, 3019–3030 (2003). [CrossRef]
16. Lempers VJ, Alffenaar JW, Touw DJ, Burger DM, Uges DR, Aarnoutse RE BR. Five year results of an international proficiency testing programme for measurement of antifungal drug concentrations. J Antimicrob Chemother 69, 2988–2994 (2014). [CrossRef]
All site content, except where otherwise noted, is licensed under a Creative Commons Attribution 4.0 License