OPEN-ACCESS PEER-REVIEWED
REVIEW ARTICLE
Vasileios Alampanos, Victoria Samanidou*, Ioannis Papadoyannis
Laboratory of Analytical Chemistry, Department of Chemistry, Aristotle University of Thessaloniki, Greece.
Journal of Applied Bioanalysis. Vol.5. No.1. pages 9-17 (2019)
Published 15 January 2019. https://doi.org/10.17145/jab.19.003 | (ISSN 2405-710X)
- Abstract
- Keywords
- Introduction
- Sample preparation techniques
- Solid Phase Extraction
- Solid-phase microextraction (SPME)
- Micro-solid-phase extraction (Micro SPE: μ-SPE)
- Dispersive micro-solid-phase extraction (DMSPE)
- Magnetic solid—phase extraction (MSPE)
- Microextraction by packed sorbent (MEPS)
- Figures and Tables
- Stir bar sorptive extraction (SBSE)
- Spin column Extraction (SCE)
- Liquid phase extraction
- Liquid-phase microextraction (LPME)
- Molecularly imprinted polymers (MIPs) for sample preparation
- Molecularly imprinted solid-phase extraction (MISPE)
- Molecularly imprinted solid-phase micro-extraction (MI-SPME)
- Conclusion and perspectives
- References
Correspondence:
Samanidou V. . Laboratory of Analytical Chemistry, Department of Chemistry, Aristotle University of Thessaloniki. Thessaloniki, Greece. Phone: +30 2310997698; Fax: +30 2310997719.
Citation:
Alampanos V, Samanidou V, Papadoyannis I. Trends in sample preparation for the HPLC determination of penicillins in bio-fluids.
J Appl Bioanal 5(1), 9-17 (2019).
Open-access and Copyright:
©2019 Alampanos V et al. This article is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Funding/Manuscript writing assistance:
The authors have no financial support or funding to report and they also declare that no writing assistance was utilized in the production of this article.
Competing interest:
The authors have declared that no competing interest exist.
Article history:
Received: 30 October 2018, Revised 08 January 2019, Accepted 14 January 2019.
Abstract
Penicillin antibiotics are widely used for antibacterial treatment. Quantitative determination of these drugs in bio-fluids is constantly under a great need. HPLC is the more common analytical technique for this purpose. During the last decades, Green Analytical Chemistry is on the rise and is proven a new tendency. Sample preparation, as a crucial step of the analytical procedure, is strongly affected and determined by this new scientific perspective. Therefore, a variety of new microextraction techniques have been developed, which can be combined with the chromatographic determination of biofluids. In this review, current trends and methods of sample preparation are presented, which are appropriate to be used in order to extract penicillin antibiotics from biological samples prior to their HPLC separation. These methods are compatible with the principles of green analytical chemistry, which is an extreme necessity of our era, for both environmental and economic reasons. The evolution and establishment of these microextraction analytical techniques for sample preparation constitute a significant field of modern research due to their importance in the whole analytical procedure. Bioanalytical applications are set in the spotlight.
Keywords
Sample preparation, penicillin, biofluids, biological samples, HPLC determination.
Introduction
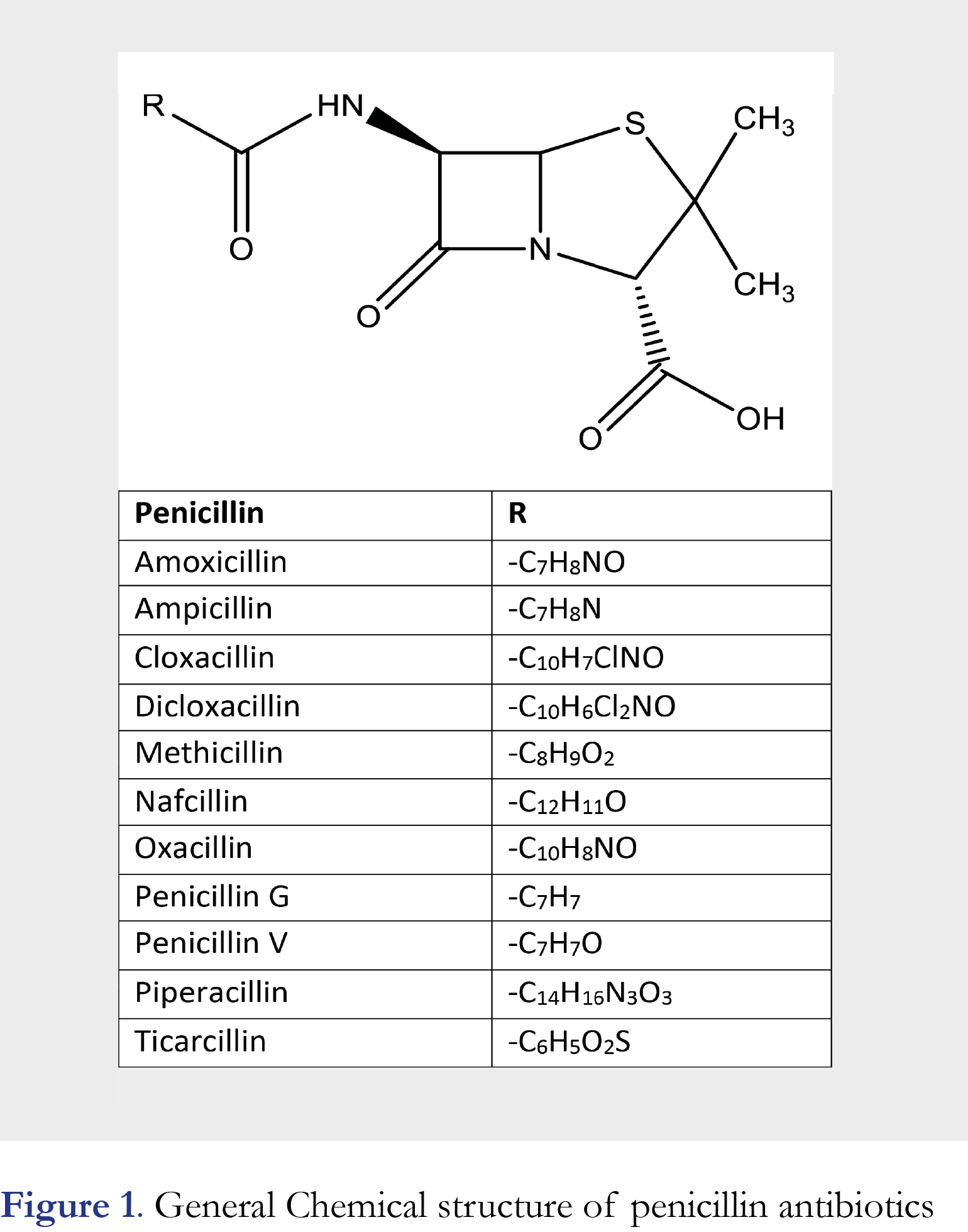
Penicillins constitute a category of bicyclic organic compounds which are characterized by the presence of a β-lactam ring fused with a thiazoline ring, as shown in Figure 1. They appear to be highly effective against bacterial infections. Therefore antibiotics are extensively used by humans and animals by ingestion. These drugs inhibits the enzymes involved in the biosynthesis of the peptidoglycan in the cell wall. the enzymes involved in the biosynthesis of the peptidoglycan in the cell wall. Penicillins are classified, based on the mode of their activity, or according to their efficacy against bacterial β-lactamases, so they can be used as narrow-spectrum antibiotics. There is constantly a great interest for the quantitative determination of penicillins in biofluids for various reasons as for example, the knowledge of the time above the minimum inhibitory concentration (t > MIC) the most determinant parameter, researching the curative efficacy of β-lactam antibiotics in therapeutic drug monitoring (TDM) [1-2].
The occurrence of penicillin residues is usually observed in food samples, water samples, and biological samples. In the past few years, the overuse of antibiotics is a constantly increasing phenomenon which constitutes a significant problem for public health. As for food products, remarkable research and articles have been published so far. In addition, agreements between states such as directions and measures from the European Union define the Maximum Residue Limits (MRL) for each product and establish the control measures and the alert plans to be applied for the detection of various special substances. Due to the excessive use, penicillins are considered emerging pollutants, too [3-8].
However, this review focuses on the biological samples, namely biofluids, which commonly include whole blood, serum, plasma, urine, saliva, breast milk, sweat, cerebrospinal fluid, gastric fluid, exhaled breath, and tissue samples (i.e., hair, nail, skin, bone, muscle). For this purpose, the most suitable analytical technique is defined by the complexity of the matrices as well as the low concentration levels, which demand high sensitivity, and extremely selective methods for the accurate determination of penicillins. High performance liquid chromatography (HPLC) has been established as the most suitable one, combined with different detection techniques, mainly with mass spectrometry (MS) or UV-VIS (200-800 nm) detection by using diode-array detection (DAD) [9-10].
The huge development of innovative analytical instrumentation certainly improves the analytical procedure. However, most of the time, in order to improve the detection ability, the selectivity, and sensitivity and in order to protect the analytical instrument from possible damage, a sample preparation step is necessary. This step is one of the most crucial and important steps for the whole procedure. Sample preparation must ensure a quantitative recovery of target analytes avoiding contamination and providing matrix isolation leading to a reduction of interferences and matrix effects during the measurement step. Accordingly, when it is used, this step enhances accuracy, precision, robustness, selectivity, and sensitivity of the analysis and is necessary to preconcentrate the analytes of interest and provide reliable results. An important restricting factor of this is the matrix effects because biological matrices are very complicated and usually contain proteins, lipids, drugs, salts, acids, bases and various other organic and inorganic compounds with similar chemical behavior and properties to the analytes, which can impede the measurements for example with causing a deviation from the actual value of an analyte concentration Undoubtedly, sample preparation is the most determinative factor regarding the efficacy of analytical methods. Consequently, regarding biological samples, sample preparation prior to chemical analysis is required because the target analytes are present in low concentrations levels, often lower than the limits of detection of the analytical instrument. Moreover, most biological samples are not compatible with the analytical instruments and they are too complex for direct analysis. Therefore, research articles and reviews have been reported which represent some traditional methods for the sample preparation of biofluids [11-12].
Nevertheless, taking into consideration the principles that result from the Green Analytical Chemistry (GAC) and obviously the need of more environmental-friendly perspectives, contemporary microextraction techniques should receive all of our interest and must replace, more than ever, other ways of sample preparation. In the center of GAC stands this step, as the most challenging and the most contributing part, in which depends the harmonization of the examined technique with GAC philosophy, which refers to the role of analytical chemists in making clever combination of environment-friendly and cheap methodologies and recommended in 12 principles [13-15]. So then, the requested goals for all these microextraction methods are the utilization of smaller volumes of liquid solvents, the minimization of sample number and size, the quantitative recovery of target analytes, the matrix isolation and, as a result, the avoidance of interferences and matrix effects to the next steps, reduction of the sample treatment steps, less consumption of hazardous reagents, organic solvents and energy, the maximization of safety and finally the significant increment of the analytes` concentration [16].
All the above-mentioned characteristics also constitute the advantages of using microextraction methods in biofluids against classic procedures, such as Liquid-Liquid Extraction (LLE) and Solid Phase Extraction (SPE). Additionally, contemporary microextraction techniques seem to have better coupling in general with the instrumental equipment. So then, microextraction techniques, few years after their first proposal, since solid-phase microextraction (SPME) was developed by Pawliszyn and his coworkers in 1990, have already been established and continue to find more and more increasing applications [17-18].
Subsequently, in this mini-review, some contemporary proper microextraction techniques are presented coming from and complying with the green analytical chemistry principals. They are mainly based on solid adsorbents and less on liquid phase and are the modern trends in sample preparation of antibiotics such as penicillins in biofluids. Either they have been already been tested in the HPLC determination of penicillins in biofluids successfully, either they have all the suitable characteristics to be used for this purpose. In any case, more research and experimental procedures should be tried due to the necessity of the present time for establishing new, greener methods.
Sample preparation techniques
Solid Phase Extraction
Solid phase extraction is a classic and widely used extraction technique for biofluids. This technique can be applied in three ways, namely the manual, the semi-automatic or the automatic way. In any case of application, SPE compared with LLE reduce the volumes of the used organic solvents. In addition, the possibility of emulsion formation is strongly limited. Despite of these, SPE demands an extensive procedure and is time-consuming in comparison with modern techniques such as SPME and Micro SPE, which eliminate the sample pretreatment steps and analysis time. In order to overcome the disadvantages, novel solid-phase microextraction methods have been developed.
Solid-phase microextraction (SPME)
As was mentioned before, SPME was presented by Pawliszyn and his co-workers as a suitable solvent-free technique. Nowadays, it is used extensively, and it complies with GAC with plenty of applications. This technique seems to gain growing attention owing to the proven advantages such as the short extraction time, simple equipment, effectiveness, cost minimization, high sensitivity (possibility of determination at the ppt level) [17]. SPME can be used with all the matrices belong to the category of biofluids and has a simple coupling with different instrumental techniques, usually with liquid chromatography (LC), gas chromatography (GC), high-performance liquid chromatography (HPLC) in the on-line or off-line modes. In addition, it is proper for a great amount of organic and inorganic substances in any one of the three kinds of physical states. The mode of operation of SPME is based on the diffusion of the analytes from the sample matrix to the solid sorbents. It includes two stages. It starts with the adsorption of analytes into the sorbent phase and then the solvent or thermal desorption of analytes from the sorbent phase follows prior to their introduction into the HPLC. The choice of stationary phase, which should have the highest affinity to the desirable analytes, presents a high degree of difficulty and is the main problem that an analytical chemist should deal with, especially due to the lack of commercially available stationary phases. This fact constitutes the only disadvantage of this technique. The mainly used materials are restricted to divinylbenzene (DVB), polydimethylsiloxane (PDMS), carboxen (CAR), polyethylene glycol (PEG) and Carbowax (CW). Therefore, a significant number of new nanomaterials or modified nanomaterials such as carbon nanotubes, graphene and graphene oxide, molecularly-imprinted polymers, metallic nanoparticles have emerged and tend to be used more extensively. Contemporary variations of the method like fiber SPME, in-tube SPME, in vivo SPME, in-tip fiber SPME, immunoaffinity solid phase microextraction have been developed and tested [11, 16,18-21]. Immunoaffinity solid phase microextraction method has been applied for the identification of penicillin-binding protein 2a (PBP2a) [22]. Subsequently, various methods based on SPME are presented.
Micro-solid-phase extraction (Micro SPE: μ-SPE)
Micro-solid-phase extraction is a novel pre-concentration and extraction technique, which could possibly be a useful alternative to multistep SPE. Its first proposal was in 2006 by Basheer and coworkers and this technique is also known as porous membrane-protected SPE [24-25]. This approach gives great results in the elaboration of complex biological samples, such as ovarian cyst fluid, plasma, urine and human cerebrospinal fluid.[23-25] Significant benefits include the high extraction efficiency (recovery from 74 to 101.1% based on the matrix and the specific analyte), the extraction and concentration of the analytes in a one single step, the less time consumption (convenient for daily operation), the minimized usage of solvent and the high enrichment. Of course, it is also environmentally friendly. In short, the process of this technique includes the package of a small amount of sorbent material in small sorbent bags (1–4 cm2) made of porous polypropylene (PP) membrane and its edges are heat sealed after the package of the sorbent. Thereinafter, follows the diffusion of the analytes through the membrane’s pores and their retention in the solid sorbent phase without requiring vacuum either for loading or elution [23-25].
Dispersive micro-solid-phase extraction (DMSPE)
DMSPE is the dispersion of some micrograms of an extracting sorbent in the sample solution and the adsorption of the analytes on the sorbent particles. Afterwards, the centrifugation of the created suspension follows. In the end, the analytes are eluted with the usage of the suitable organic solvent. It can be characterized as a simple and fast microextraction technique highly compatible with HPLC. Moreover, it offers high extraction performance (recovery from 78 to 119 % based on the matrix and the specific analyte) in combination with an easy and fast procedure. All the materials complied with DMSPE should have large surface area able to guarantee fast, quantitative sorption and elution. In addition, these materials should have high capacity and dispersibility in liquid samples. Examples of this kind of materials are the carbon nanomaterials like carbon nanotubes (CNTs), graphene and fullerene and inorganic nanoparticles like magnetic nanoparticles (MNPs). The application of DMSPE in various biofluids has all the desirable results, which are in general the high recovery values with low sorbent and organic solvent requirements and fast procedure. All these developed methods are cheap in comparison with other techniques, low time-consuming and fast compared with classic SPE and of course compatible with GAC [26].
Magnetic solid—phase extraction (MSPE)
This method has been established as an already tested one by Safaríková and Safarík in 1999 And has been widely used in many fields, including bioanalysis [31]. It shows convenient results, used as a preconcentration step of different organic and inorganic analytes and their isolation from complex biological samples like urine, serum, plasma, blood, hair. According to this method, the magnetic sorbent is scattered into the sample solution, which includes the targeted analytes that are adsorbed in the surface of the NPs. So then, the analytes are eluted with the proper solvent. It is considered to be a green, fast and clean method, which does not waste time, energy and hazardous organic solvents. It has all the needed features, such as high recovery values (from 82.5 to 103 % based on the matrix and the specific analyte), speed, less steps of extraction procedure harmonization to GAC in order to gain more ground in the field of bioanalysis. Nanoparticles containing Fe3O4 are the most commonly used materials in MSPE due to their low cost and have low toxicity. A great interest has also arisen in carbon materials. In comparison with the traditional SPE, this method demands less analysis time and eliminates the steps of the extraction process. At the same time, the waste is restricted, and it can be used for any kind of the complex biological samples like urine, serum, plasma, blood, hair, offering high sensitivity and recovery [16, 27-29].
Microextraction by packed sorbent (MEPS)
MEPS is a very characteristic technique, which reflects the influence of Green Analytical Chemistry in sample preparation. Essentially, it is resulted from the miniaturization and the size restriction of the classic SPE. As sorbent materials, they could be used for all the commercially available materials for SPE, new polymeric materials like polystyrene and surely reversed phase, normal phase, ion exchange and organic monolithic sorbents. In short, the sequence of the procedure is sorbent conditioning, sample loading, washing and analytes eluting. So then, MEPS is a simple, fast and inexpensive green analytical technique which requires a reduced amount of sample in comparison with classic SPE in general less than 350 μL and obviously small volumes of sorbents and solvents at the size of μL. More specifically, washing and elution steps can be carried out with 20–50 μL of organic solvents and 1–4 mg of reusable sorbent material are enough for efficient analyte extraction. In addition, the extraction of the analytes takes part in one single device. All the steps seem to have suitable repetition, while the whole procedure have excellent coupling with LC analysis in either automatic or semi-automatic ways. Finally, MEPS has undoubted efficacy towards in bioanalytical applications [16, 30-31].
Figures and Tables
[Click to enlarge]
Stir bar sorptive extraction (SBSE)
Stir bar sorptive extraction is a modern preparation technique which has been firstly introduced by Sandra and coworkers in 1999 [32]. It is considered a very hopeful method, notably suitable for volatile and semi-volatile compounds. As the aforementioned techniques, it relies on the initial absorption and the following adsorption of the analytes. This procedure takes place on the surface of a proper magnetic stirring bar, which is coated with a thin layer of the suitable sorbent. In the end, stir bar is carefully dried with soft tissue. Essentially, a liquid sample is simply stirred with this appropriate bar. Therefore, it is an easy extraction, rapid, environmentally friendly, green and low-cost method. The materials coating the stir bars are mainly polydimethylsiloxane (PDMS), which are easily provided and commercially available in the market. The retention participates owning to the development of Van der Waals and hydrogen bonds between the oxygen of PDMS and the analytes. The only noticeable disadvantage of this technique is the fact that the polymeric (PDMS) overlay of stir bars are non-polar and so can be properly used for hydrophobic interactions. As a result, it is not efficient for polar compounds. This restriction could be overcome with the utilization of specialized materials like MIPs (molecularly imprinted polymer). However, this technique shows great efficacy in plenty of applications in biological matrices, like urine, plasma, and serum, especially for volatile compounds [33, 34].
Spin column Extraction (SCE)
Namera and his coworkers firstly introduced the Spin Column Extraction in 2008. It is based on the package of a monolithic silica disk into a spin-column. Substantially, all the stages of the process which as usual are sample loading, washing, and analytes elution, are carried out only with the centrifugation of the column. Therefore, it is a simple, fast, green technique with the significant advantage that many samples can be worked on simultaneously. This feature is very important for biofluids [34, 35].
Liquid phase extraction
The classic liquid phase extraction (LLE) used to be the most commonly chosen and tried a sample preparation technique for the extraction of various chemical compounds. It can be easily observed by the literature and the published research articles that are still used nowadays very often. Despite all these, since Green Analytical Chemistry has arisen, LLE started to be considered as a time-consuming, wasteful, and hostile to the environment technique. Therefore, similarly to the case of SPE, some novel liquid phase microextraction methods have been introduced in order to replace the old techniques. Below in the text, some of them are briefly presented. They are proven efficient or have all the necessary features to be useful for the extraction of substances like penicillins [11, 37].
Liquid-phase microextraction (LPME)
Liquid-phase micro-extraction derived from the classic LLE, but it is simpler, faster, miniaturized, environmentally friendly and automated. The mode of the procedure includes the extraction of the analytes from water samples into small amounts like drops at the size of microliters of non-miscible with water solvents. There are some modifications of LPME, which are mainly Single-drop microextraction (SDME), Hollow fiber liquid-phase microextraction (HF-LPME), Dispersive liquid-liquid microextraction (DLLME), Single drop microextraction SDME [16,37].
Molecularly imprinted polymers (MIPs) for sample preparation
MIPs are synthetic polymeric chemical compounds with specific and efficient recognition abilities in order to catch and bound target analytes in preference to other existent compounds. It is possible, owing to the utilization of a proper template molecule during the synthesis of MIPs. The molecules that are used, are chosen after taking carefully into consideration the way they interact and bind with the targeted molecules. MIPs provide selectivity and sensitivity. Moreover, they are stable, robust and resistant in a wide range of condition of temperature, pH or used solvent. They can be easily observed, with a sight into their mode of action, that there is an imitation behavior of action in natural interactions, this time with the avoidance of stability restrictions. On account of their properties to be excellent selective materials they can be used as a sorbent in solid phase microextraction techniques such as SPE and SPME and its variations (i.e. MSPME) and surely in the sample preparation of biofluids [38]. Other formats of these valuable materials have been proposed with the development of MIPs coated materials, such as in the case of stir bar sorptive extraction, MEPs.
Molecularly imprinted solid-phase extraction (MISPE)
MISPE can be applied in two main modes which are the classic offline SPE procedure as well as some variant of the online protocol. Up to date, numerous works, researches, and papers about the utilization of MISPE and MIPs for the extraction of the analytes have been published. Undoubtfully, this method is considered very efficient for the isolation of antibiotics like penicillins and relevant compounds in biofluids. The first complete application was introduced in 1994 by Sellergren [9, 38-39].
Molecularly imprinted solid-phase micro-extraction (MI-SPME)
As it was mentioned before, SPME relies on the severance of the desirable analytes between the sample and the stationary phase, which is a fused silica fiber coated with the sorbents. However, this method was disadvantaged due to the restriction arose by the small range of polarity that can be covered from the commercially available fiber materials. Therefore, the application of MIPs was introduced by Mullet in 2001 [40]. MIPs can be involved by packing a capillary for in-tube SPME. Nevertheless, this procedure demands more instrumental equipment. So then, the researchers’ attention was strained to the preparation of silica fibers coated with a MIP [9, 38-39].
Conclusion and perspectives
As it is commonly declared, sample preparation/pretreatment is the bottleneck of the whole analytical procedure and is fairly considered as the most challenging step. Obviously, sample preparation for the extraction of antibiotics like the ββ-lactams and penicillins in biofluids prior to HPLC and in general prior to liquid chromatography is always in the spotlight of applied bioanalysis. There is a wide spectrum of classic techniques which have been used. However, the rise of Green Analytical Chemistry has brought to the surface a definitely new approach to this matter. A few years now the researches have turned to more environmentally friendly and miniaturized new techniques. As we presented, these techniques mainly consist of an efficient variation of SPE and LLE. The main features that influenced this transition are the need for less extraction time, less solvent volume, less toxic organic solvents, simplicity, repeatability, low cost per analysis, low cost of equipment and automation. Some liquid extraction methods like the LPME, DLLME, and SDME are simple, fast and cost-effective, but they are difficult to automate, and their selectivity is poor. Therefore, microextraction techniques based on solid phase extraction gain more of our interest. From that approach, the future research for green microextraction techniques could focus on the development and further application of other MSPE variations, the evolvement of new sorbent materials, the utilization of fewer amounts of incorporated substances and solvents, the further miniaturization and automation, the easier on-line coupling with the instrumental equipment. Undoubtedly, in this way clinical and pharmacological research will be enhanced with more green tools for the investigation of antibiotics such as penicillins.
References
1. The Merck Veterinary Manual, Merck & Co. 2009. http://www.merckvetmanual.com/mvm/pharmacology/antibacterial_agents/penicillins.html, (Accessed 25 October 2018).
2. Commission Regulation EU No. 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off J Eur Union L15, 1–72 (2010).
3. European Commission, Commission Decision (EEC) 2002/657/EC, Off J Eur Comm, Official Journal L 221, 8 – 36 (2002).
4. European Commission, Council Directive (EEC) 96/23/EC, Offi J Eur Comm, L125, 10–32 (1996).
5. Ohmori T, Suzuki A, Niwa T et al. Simultaneous determination of eight -lactam antibiotics in human serum by liquid chromatography–tandem mass spectrometry. J Chrom B 879, 1038–1042 (2011). [CrossRef]
6. Soliman MA, Pedersen JA, Suffet IH. Rapid gas chromatography-mass spectrometry screening method for human pharmaceuticals, hormones, antioxidants and plasticizers in water. J Chrom A 1029, 223-237 (2004). [CrossRef]
7. Dıaz-Cruz MS, Lopez De Alda MJ, Barcelo D. Environmental behavior and analysis of veterinary and human drugs in soils, sediments and sludge. Trends Anal Chem 22(6), 340-351 (2003). [CrossRef]
8. Xue YJ, Gao H, Ji QC et al. Bioanalysis of drug in tissue: current status and challenges. Bioanalysis 4, 2637-2653 (2012). [CrossRef]
9. Lara FJ, Olmo-Iruela M, Cruces-Blanco C et al. Advances in the determination of b-lactam antibiotics by liquid chromatography. Trends Anal Chem 38, 52-66 (2012). [CrossRef]
10. Di Rocco M, Moloneya M, O’Beirnec T et al. Development and validation of a quantitative confirmatory method for 30 b-lactam antibiotics in bovine muscle using liquid chromatography coupled to tandem mass spectrometry. J Chrom A 1500, 121–135, (2017). [CrossRef]
11. Niu Z, Zhang W, Yu C et al. Recent advances in biological sample preparation methods coupled with chromatography, spectrometry and electrochemistry analysis techniques. Trends Anal Chem 102, 123-146 (2018). [CrossRef]
12. Novakova L, Challenges in the development of bioanalytical liquid chromatography-mass spectrometry method with emphasis on fast analysis, J Chrom A 1292, 25-37 (2013). [CrossRef]
13. Gałuszka A, Migaszewski Z, Namiesnik J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices, TrAC– Trends Anal Chem 50, 78–84 (2013). [CrossRef]
14. Turner C. Sustainable analytical chemistry—more than just being green. Pure Appl Chem 85, 2217–2229 (2013). [CrossRef]
15. Armenta S, Garrigues S, de la Guardia M. The role of green extractiontechniques in Green Analytical Chemistryl. Trends Anal Chem 71, 2–8 (2015). [CrossRef]
16. Filippou O, Bitas D, Samanidou V. Green approaches in sample preparation of bioanalytical samples prior to chromatographic. J Chrom B 1043, 44–62 (2017). [CrossRef]
17. Arthur CL, Pawliszyn J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal Chem 62, 2145–2148 (1990). [CrossRef]
18. Lord H, Pawliszyn J. Evolution of solid-phase microextraction technology, J Chrom A 885, 153-193 (2000). [CrossRef]
19. Lum TS, Tsoi YK, Leung KS. Current developments in clinical sample preconcentration prior to elemental analysis by atomic spectrometry: a comprehensive literature review. J Anal At Spectrom 29, 234-241 (2014). [CrossRef]
20. Delafiori J, Ring G, Furey A. Clinical applications of HPLC-ICP-MS element speciation: a review. Talanta 153, 306-331 (2016). [CrossRef]
21. Fernandez-Peralbo MA, Luque de Castro MD. Preparation of urine samples prior to targeted or untargeted metabolomics mass-spectrometry analysis. Trends Anal Chem 41, 75-85, (2012). [CrossRef]
22. Liu Y, Lord H, Maciazek-Jurczy M et al. Development of an immunoaffinity solid phase microextraction method for the identification of penicillin binding protein 2a. J Chrom A 1364, 64–73 (2014). [CrossRef]
23. Płotka-Wasylka J, Szczepanska N, de la Guardia M, Namiesnik J. Miniaturized solid-phase extraction techniques. Trends Anal Chem 73, 19–38 (2015). [CrossRef]
24. Lashgari M, Basheer C, Kee Lee H. Application of surfactant-templated or deredmesoporous material as sorbent in micro-solid phase extraction followed by liquid chromatography-triple quadrupole mass spectrometry for determination of perfluorinated carboxylic acids in aqueous media. Talanta 141, 200–206 (2015). [CrossRef]
25. Kanimozhi S, Basheer C et al. Application of porous membrane protectedmicro-solid-phase-extraction combined with gas chromatography-mass spectrometry for the determination of estrogens in ovarian cyst fluid samples. Anal Chim Acta 687, 56–60 (2011). [CrossRef]
26. Asgharinezhad AA, Karami S, Ebrahimzadeh H et Al. Polypyrrole/magnetic nanoparticles composite as an efficient sorbent fordispersive micro-solid-phase extraction of antidepressant drugs from biological fluids. Int J Pharm 494, 102–112 (2015). [CrossRef]
27. Safaríková M, Safarík I. Magnetic solid-phase extraction. J Magn Mater 194, 108–112 (1999). [CrossRef]
28. Płotka-Wasylka J, Szczepanska N, de la Guardia M et al. Moderntrends in solid phase extraction: new sorbent media. Trends Anal Chem 77, 23–43 (2016). [CrossRef]
29. Herrero-Latorre C, Barciela-García J, García-Martín S et al. Magnetic solid-phase extraction using carbon nanotubesas sorbents: a review. Anal Chim Acta 892, 10–26 (2015).
30. Moein MM, Abdel-Rehim A, Abdel-Rehim M. Microextraction by packed sorbent (MEPS). Trends Anal Chem 67, 34–44 (2015). [CrossRef]
31. Kole PL, Venkatesh G, Kotecha J et al. Recent advances in sample preparation techniques for effective bioanalytical methods. Biomed Chromatogr 25, 199–217, (2011). [CrossRef]
32. Baltussen E, Sandra P, David F et al. Stir bar sorptive extraction SBSE, a novel extraction technique for aqueous samples: theory and principles. J Microcolumn Sep 11(10), 737-747 (1999). [CrossRef]
33. Nogueira JM. Novel sorption-based methodologies for static microextraction analysis: a review on SBSE and related techniques. Anal Chim Acta 757, 1–10 (2012). [CrossRef]
34. Nazyropoulou C, Samanidou V. Stir bar sorptive extraction applied to the analysis of biological fluids. Bioanalysis 7, 2241–2250 (2015). [CrossRef]
35. Namera A, Nakamoto A, Nishida M et al. Extraction of amphetamines and methyl-ene dioxy amphetamines from urine using a monolithic silica disk-packed spin column and high-performance liquid chromatography-diode array detection. J Chrom A 1208,71-75 (2008). [CrossRef]
36. Saito T, Yamamoto R, Inoue S et al. Simultaneous determination of amitraz and its metabolite in human serum by monolithic silica spin column extraction and liquid chromatography-mass spectrometry. J Chrom B: Analyt Technol Biomed Life Sci, 867, 99-104 (2008). [CrossRef]
37. Han D, Row KH. Trends in liquid-phase microextraction and itsapplication to environmental and biological samples. Microchim Acta 176, 1–22 (2012). [CrossRef]
38. Turiel E, Martín-Esteban A. Molecularly imprinted polymers for sample preparation: A review. Anal Chim Acta 668, 87–99 (2010). [CrossRef]
39. Sellergren B. Imprinted dispersion polymers: A new class of easily accessible affinity stationary phases. J Chrom A 673(1), 133-141 (1994). [CrossRef]
40. Mullett WM, Lai EP. Determination of Theophylline in Serum by Molecularly Imprinted Solid-Phase Extraction with Pulsed Elution. Anal Chem 70(17), 3636-3641 (1998). [CrossRef]
All site content, except where otherwise noted, is licensed under a Creative Commons Attribution 4.0 License