OPEN-ACCESS PEER-REVIEWED
RESEARCH ARTICLE
Mariana Millan Fachi1, Bruna Carolina Lui Dias1, Jonata Augusto de Oliveria2, Rosângela Gonçalves Peccinini2, Roberto Pontarolo1, Michel Leandro de Campos1,3,*
1Pharmaceutical Sciences Post-Graduate Program, Federal University of Paraná (UFPR), Curitiba, Brazil; 2Department of Natural Active Principles and Toxicology, São Paulo State University (UNESP), School of Pharmaceutical Sciences, Araraquara, Brazil; 3Health Sciences Institute, Federal University of Mato Grosso (UFMT), Sinop –MT, Brazil.
Journal of Applied Bioanalysis. Vol. 9 pages e23001 (2023).
Published online 10 February 2023. https://doi.org/10.17145/jab.23.001 | (ISSN 2405-710X)
*Correspondence:
Michel Leandro de Campos .
Health Sciences Institute Federal University of Mato Grosso, Av. Alexandre Ferronato, 1200 Sinop, Mato Grosso, Brazil; Phone: +55 6635333194.
Citation:
Fachi MM, Lui Dias BC, de Oliveria JA, Gonçalves Peccinini R, Pontarolo R, de Campos ML. Pharmacokinetic Profile of Metformin and SGLT2 Inhibitors alone and in Combination: a Pharmacokinetic Study in Wistar Rats. J Appl Bioanal 9, e23001 (2023).
Open access and Copyright:
©2023 Fachi M et al. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Funding/Manuscript writing assistance:
The authors have declared that this work was supported by financial support and that no writing assistance was utilized in the production of this article.
Competing interests:
The authors have declared that no competing interest exist
Article History:
First draft submitted: October 31, 2022; Revised: January 31, 2023; Accepted for publication: February 01, 2023.
Summary
Objective: To achieve glycemic control, a combination of drugs is eventually necessary, especially the dual therapy of SGLT2 inhibitors with metformin. Despite the value of combination therapy, understanding the pharmacokinetic properties is critical. Therefore, this study aimed to conduct the combined and isolated administration of hypoglycemic drugs to understand their pharmacokinetic properties.
Methodology: The study was performed by gavage in twenty-five rats that were divided into five groups: metformin alone (60 mg/kg), canagliflozin alone 20 mg/kg, canagliflozin and metformin (20 mg/kg and 60 mg/kg, respectively), dapagliflozin alone 2 mg/kg, and dapagliflozin and metformin (2 mg/kg and 60 mg/kg, respectively). Blood samples were collected between 0.25 and 36 hours postdose and quantified by an HPLC-MS/MS method.
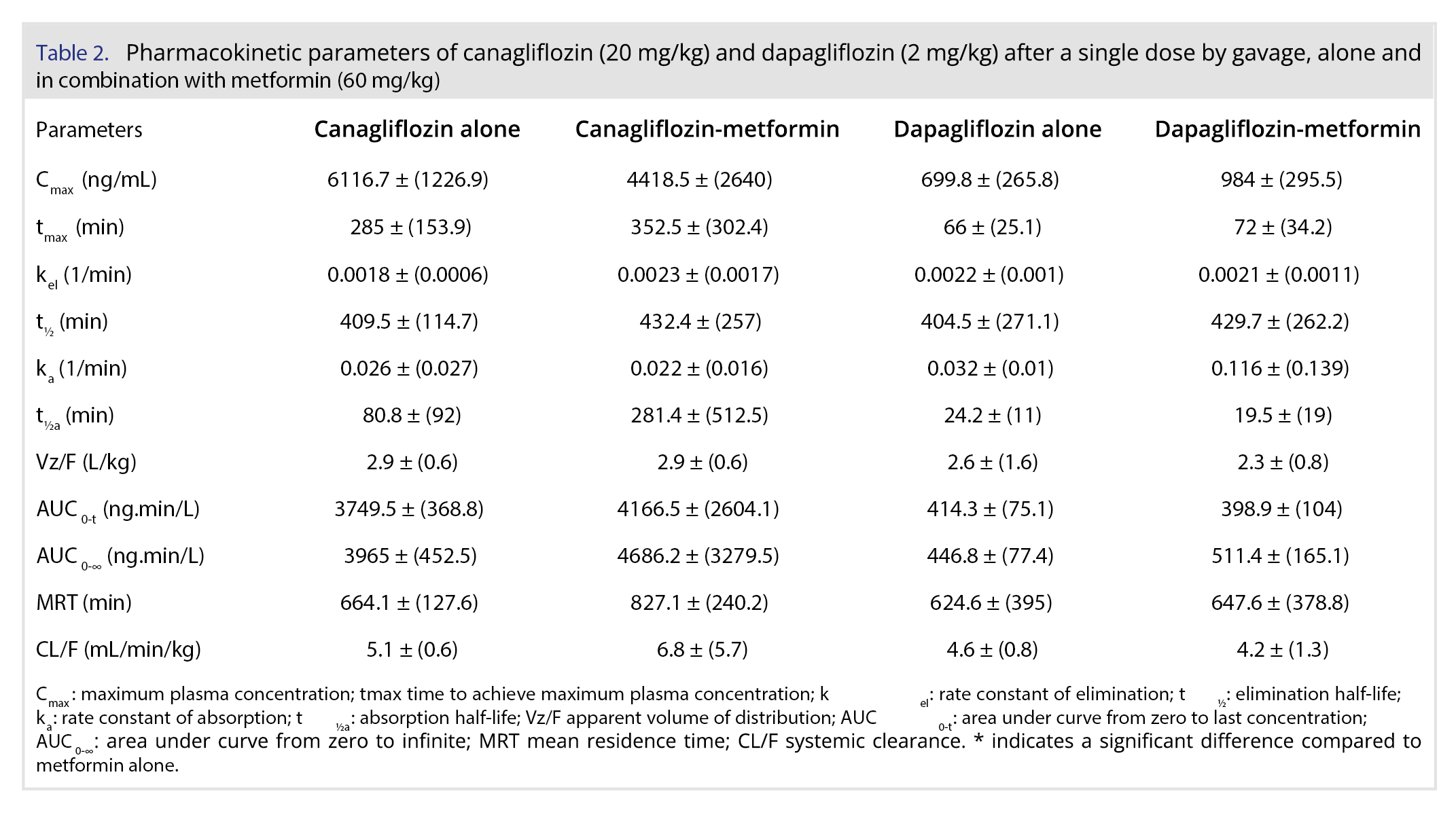
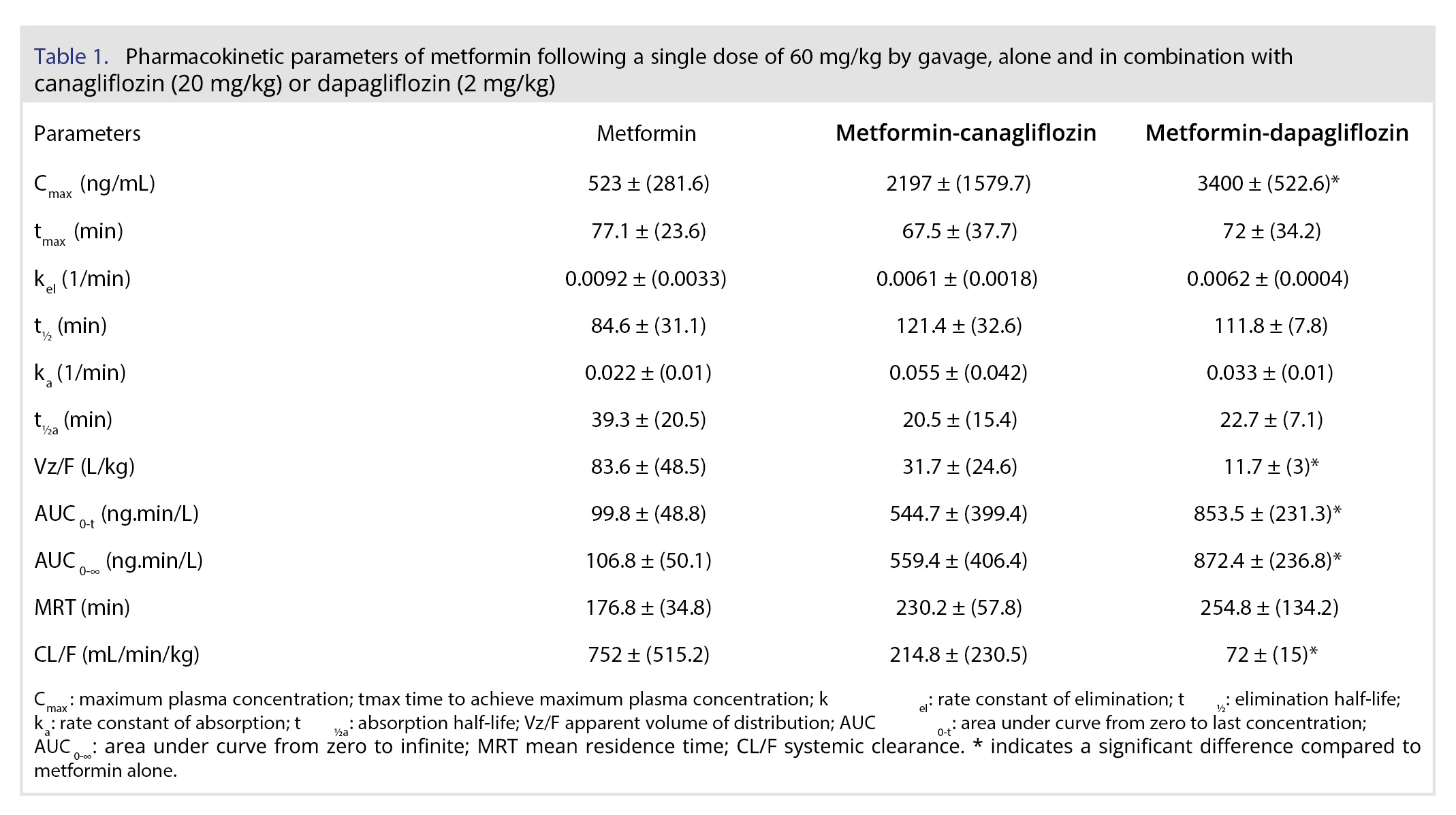
Results: The metformin pharmacokinetics showed values lower than those from literature, but the most relevant result was a significant change in Cmax (3400 ng/mL), AUC (872.4 ng.min/L) and CL/F (72 mL/min/kg) in the metformin with dapagliflozin group compared to metformin alone Cmax (523 ng/mL), AUC (106.8 ng.min/L) and CL/F (752 mL/min/kg). For canagliflozin, the Cmax of 6116.7 ng/mL observed in our study was similar to that observed in literature, while the clearance (5.1 mL/min/kg) was higher than that of literature, which was 3.5 mL/min/kg. Clearance of dapagliflozin CL/F was reported as 3.33 mL/min/kg, while our result was 4.6 mL/min/kg. The same study also published dapagliflozin half-life and MRT, which were slightly lower than our findings. In general, the parameters of canagliflozin and dapagliflozin were similar to the literature and did not change with simultaneous administration with metformin.
Conclusion: Dapagliflozin significantly changed the pharmacokinetic disposition of metformin, while metformin coadministration had no influence on the pharmacokinetics of SGLT2 inhibitors.
1.0 Introduction
Diabetes mellitus (DM) type 2 is a metabolic disorder characterized by chronic hyperglycemia resulting from defects in insulin action and/or secretion [1]. Since monotherapy may be insufficient to maintain glycemic control, patients may require combined therapies. Among the combination treatments, metformin has been used with a sodium/glucose cotransporter inhibitor 2 (SGLT2), such as canagliflozin and dapagliflozin [2-4]. The chemical structure of these drugs is shown in Figure 1.
Metformin acts primarily by reducing hepatic neoglycogenesis, inhibiting intestinal glucose uptake and improving peripheral insulin sensitivity [4-7]. Its usual oral dosage is 250 to 2550 mg/day for DM2 treatment, and in the absence of reduced renal function, peak plasma levels of metformin do not exceed 15-20 μM, while minimum values are between 1-5 μM [3, 4, 6].
Otherwise, SGLT2 inhibitors have as a mechanism of action the decrease in glucose reabsorption in the proximal renal tubules through the inhibition of sodium and glucose cotransport. The kidneys filter 160 to 180 g of glucose daily, and the SGLT2 cocarrier reabsorbs 90% of this value. Thus, this class of antidiabetics promotes glycosuria by altering the normal process of glucose resorption, and consequently, there is a decrease in plasma glucose. The usual daily dose varies by drug and is 5 mg to 10 mg for dapagliflozin and 100 to 300 mg/day for canagliflozin [8, 9].
Metformin and SGLT2 inhibitors have different and complementary mechanisms of action in improving glycemic control. Combination therapy of SGLT2 inhibitors based on the noninsulin hypoglycemic mechanism contributes to metformin improving the therapeutic outcome and reducing the incidence of hypoglycemia risk [10-12]. However, a drug’s effect is associated with its concentration at the site of action, so monitoring this concentration is critical. Previous studies have already shown that there are some drugs that compromise the serum concentration of metformin mainly due to competition with organic cation transporters, resulting in a high risk of metformin-associated lactic acidosis [13]. Thus, the importance of carrying out pharmacokinetic studies is highlighted, as they allow the correlation of drug concentrations with pharmacological responses, allowing clinicians to apply pharmacokinetic principles to patients’ real situations. Thus, it is possible to establish general and individual dosages and recognize factors that may compromise the efficacy and safety of the drug [14].
Therefore, this study aimed to perform the combined and isolated administration of hypoglycemic drugs, followed by the evaluation of their pharmacokinetic profiles to better understand their kinetic disposition and any potential pharmacokinetic drug-drug interaction.
2.0 Materials and Methods
2.1 Animals
Protocols for the animal studies were approved by the Research Ethics Committee of the School of Pharmaceutical Sciences, UNESP, Araraquara, Brazil (CEUA/FCF/Car 13/2017). For the preclinical pharmacokinetic study, twenty-five rats weighing approximately 250 g were used. These were divided into the following groups (each group consisted of 5 animals): metformin alone (60 mg/kg), canagliflozin alone 20 mg/kg, canagliflozin and metformin (20 mg/kg and 60 mg/kg, respectively), dapagliflozin alone 2 mg/kg, and dapagliflozin and metformin (2 mg/kg and 60 mg/kg, respectively). Doses were calculated based on allometric scaling with an ordinary dose of metformin and high doses of SGLT2 inhibitors. During the experiment, they were kept under controlled conditions of temperature (23 ± 1°C), humidity (55 ± 5%) and light (cycle 12/12 h, lights on at 07 h) and with balanced rations and water ad libitum. The experiment was performed in the light phase. Four hundred microliters of blood were collected at 0.25, 0.5, 1, 1.5, 2, 4, 6, 8, 12, 24, and 36 hours post-dose into tubes containing heparin. The plasma was separated by centrifugation at 3000×g for 10 min, and then, the samples were aliquoted at 200 µL and stored at -40°C until analysis was carried out. For collection, the animals were submitted to surgery to implant cannulas in the femoral artery [15].
Sample preparation was executed based on the protein precipitation technique mentioned by Dias et al. [16]. Therefore, an aliquot of 200 µL of blank plasma was spiked with 980 µL of acetonitrile (containing 0.1% formic acid). Thus, the sample was mix for 3 minutes and centrifuged (Eppendorf refrigerated centrifuge, model 5810-R, Hamburg, Germany) for 15 minutes at 4°C and 14000 rpm. Then, 700 µL of the supernatant was separated and subjected to complete drying (centrivap-Labconco, Kansas City, USA). Thus, the sample was resuspended in 200 µL of acetonitrile-water (50:50, v/v) containing 1 mM ammonium formate and 0.1% formic acid, and was mixed in vortex for 10 minutes. Finally, each samples was injected into the HPLC-MS/MS system.
2.2 HPLC-MS Analysis
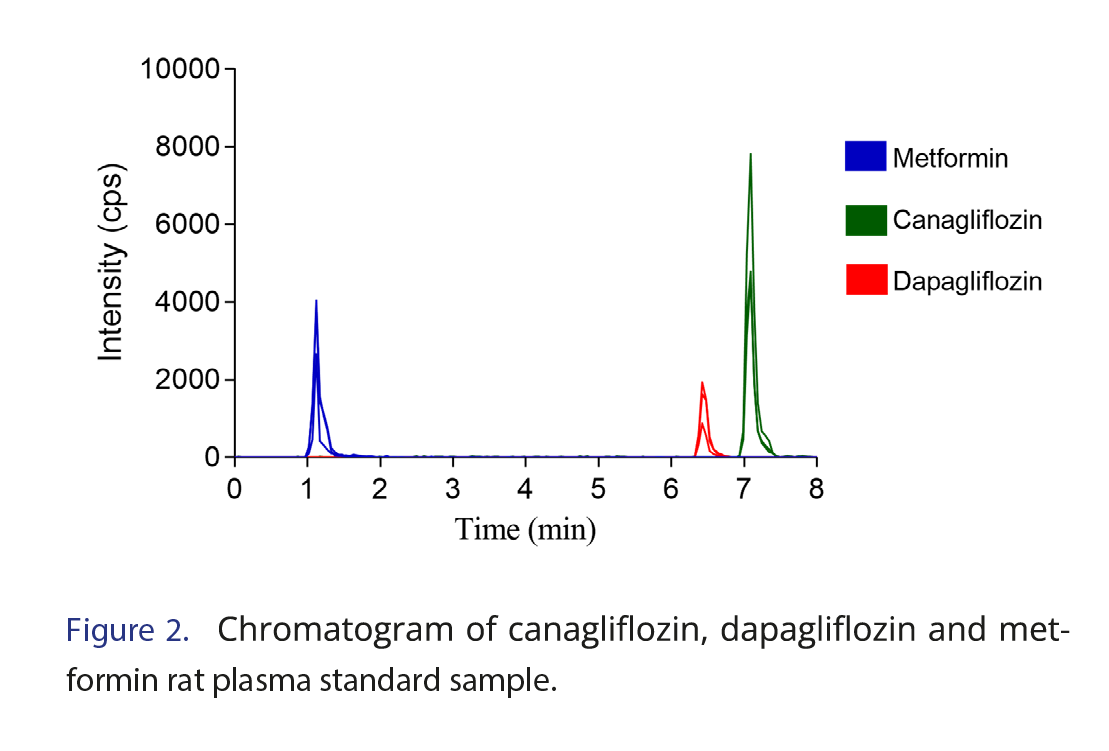
The method developed and validated by Dias et al. was used for the sample analysis [16]. The method was developed and validated on an Agilent 1200 HPLC System with a binary pump model coupled to an Applied Biosystems API 3200 triple quadrupole MS/MS with electrospray ionization in positive ion mode. Chromatographic separation was performed using an Xbridge C18 (10 x 2.1 mm, 5 µm) guard column and Xbridge C18 column (50 x 2.1 mm, 5 µm) maintained at 35°C as stationary phases and water and acetonitrile (both containing 1 mM ammonium and 0.1% formic acid) in gradient mode as the mobile phase. The chromatographic separation is demonstrated in Figure 2. Quantification was conducted in multiple reaction monitoring mode using m/z 130.1→71.1 for metformin, m/z 462.0→ 191.2 for canagliflozin, and m/z 426.1→167.1 for dapagliflozin. The method was validated according to Anvisa and the FDA [17, 18]. During validation, the following parameters were tested, with the following values achieved. Lower limit of quantification (LLOQ) has shown adequate accuracy at 10 ng/mL for dapagliflozin and 25 ng/mL for canagliflozin and metformin. Satisfactory recoveries (67.12–95.20%) were achieved for all compounds. Also, the analytes were stable in plasma (short-term temperature stability), post-preparative stability and the long-term stability test) and in solution (after 6 h at 25°C and for 72 h when stored at 4°C). It is important to highlight that as there was a change in the sample matrix (from human plasma to rat plasma), a partial validation of the previously published method [16] was carried out, as suggested by the FDA and ANVISA [17,18], testing the following analytical parameters in the new matrix: selectivity, calibration curve, precision and accuracy. In selectivity the noise values do not exceed 20% of the LLOQ concentration. Calibration curve of the method proved to be linear (r>0.99) between 25–5000 ng/mL for canagliflozin and metformin and 10–400 ng/mL for dapagliflozin for the new matrix. Accuracy and precision resulted in standard deviation and relative error both lower than 15% for quality control samples, also, there was no matrix effect.
2.3 Pharmacokinetic Analysis
Pharmacokinetic parameters were calculated by logarithmic curves of plasma concentration x time. Each animal resulted in a complete pharmacokinetic profile. Compartmental analysis was performed using Pkanalix from Monolix Suite 2021R2 (Lixoft®, Batiment D, Antony, France). A one-compartment model with first-order absorption best fit the data and resulted in the elimination (kel) and absorption (ka) constants of the model. The elimination half-life (t½) and absorption half-life were calculated by the formula 0.693/kel or ka. The area under the curve from zero to the last concentration using the trapezoidal linear up log down rule was also calculated using Pkanalix. The area under the curve from zero to infinity (AUC0–∞) was calculated by the formula AUC0–t plus the last concentration divided by kel. The area under the moment curve (AUMC) was calculated by the statistical moments method and used to determine the mean residence time (MRT) as MRT=AUMC0-∞/AUC0–∞. The clearance (Cl/F) and the volume of distribution (Vz/F) were determined by the equations Cl/F = dose/AUC0–∞ and Vz/F = Cl/kel. The maximum plasma drug concentration (Cmax) was obtained directly from the experimental data, as was the time of the occurrence of Cmax (tmax).
2.4 Statistical Analysis
Pharmacokinetic parameters are presented as the mean and standard deviation. Pharmacokinetic parameters were compared to identify the influence of the association on the pharmacokinetic profile of each drug administered alone. All comparisons were made considering α=0.05. The comparison for the metformin pharmacokinetic parameters was made by the Kruskal-Wallis test followed by Dunn’s test with the metformin alone group as a control. The Mann-Whitney test was used to compare the pharmacokinetic parameters of canagliflozin alone or dapagliflozin alone against their respective metformin concomitant administration groups. GraphPad Prism software (Version 7, GraphPad Software®, San Diego, CA, USA) was used for the statistical analysis.
3.0 Results and Discussion
Many times, diabetes management with reduction of glycemic levels may not be properly addressed with a drug alone, such as metformin, inevitably requiring multiple anti-diabetic agents to achieve glycemic control. Therefore, the selective and reversible inhibition of the SLGT2 transporter can be used to reduce glycemic levels by limiting glucose reabsorption in the kidneys. Moreover, the mechanism of SGLT2 inhibitors is not dependent on insulin, and they have no interreference with beta pancreatic cell function or the degree of insulin resistance [11, 12, 19, 20].
Figures and Tables
[Click to enlarge]
3.1 Metformin Pharmacokinetics
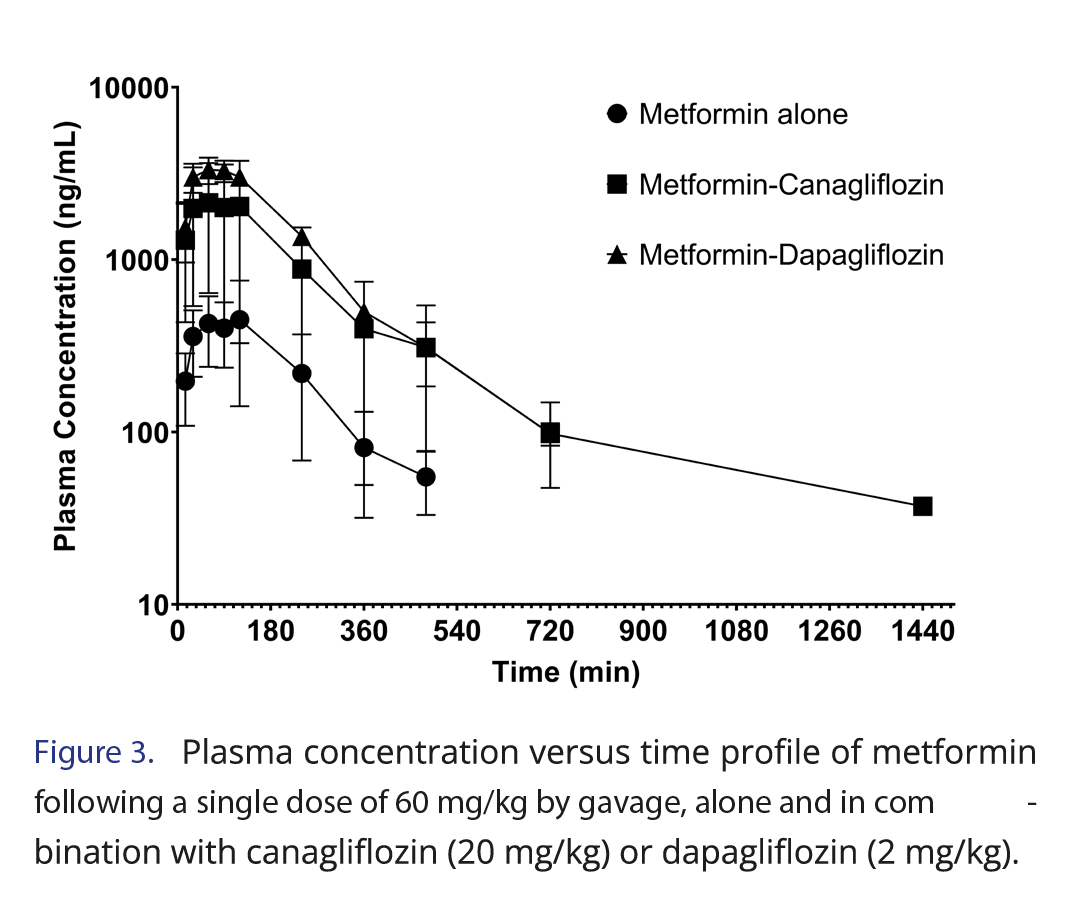
This work evaluated the potential pharmacokinetic drug-drug interaction between SGLT2 inhibitors (canagliflozin and dapagliflozin) and metformin. The results showed that canagliflozin and dapagliflozin, when administered in combination with metformin, increased metformin concentrations (Figure 3). As typical for pharmacokinetic drug-drug interactions, these have to be related to one of the pharmacokinetic processes: absorption, distribution, metabolism and excretion [21].
In humans, the absorption of metformin is incomplete (F: 55 ± 16%) with a peak concentration at 3 hours. The maximum concentration was approximately 1 mg/L after the 500 mg dose and 3 mg/L after the 1.5 g dose, indicating a proportional change in concentration after changes in dose. Its elimination half-life is approximately 20 hours [22]. Choi and coauthors [23] evaluated the pharmacokinetics of metformin in rats, and the bioavailability of metformin was approximately 30% in the dose range of 20-200 mg/kg. Our values of Cmax, AUC and elimination half-life of the metformin alone group (Table 1) were low compared to their values. It should be noted that there is great interindividual variability in the pharmacokinetics of metformin, as measured by differences in the minimum plasma concentration of metformin at steady state, ranging from 54 to 4133 ng/mL [24].
Metformin exposition parameters, such as Cmax and AUC, were significantly higher after concomitant administration of dapagliflozin. Once this difference also appeared for CL/F, only with oral administration, we cannot be sure if it happens due to higher absorption or less elimination. However, considering an average bioavailability of 30% in rats, as aforementioned, the 5-fold higher value in the canagliflozin group and the 8-fold higher value in the dapagliflozin group for AUC surpass the increase that could be explained by absorption, suggesting some level of interference in the elimination mechanisms. Dose-independent parameters, such as tmax, half-lives, and MRT, did not show any difference.
For the group treated with metformin combined with canagliflozin, an increase in the numbers was also observed; however, a significant difference was not observed (p>0.05). The study of Devineni and coauthors [25] investigated the effect of canagliflozin in metformin in volunteers and did not find differences, but the dose of canagliflozin was the regular 300 mg. Metformin is a highly hydrophilic drug primarily eliminated by the kidneys with no biotransformation, and clinically significant drug-drug interactions involving metformin are not common [13]. Based on our results, the combination of metformin and SGLT2 inhibitors in clinical situations where higher than usual doses of SGLT2 inhibitors are needed must be closely monitored for the side effects of metformin. This finding is crucial since maximal increases in urinary glucose excretion were seen at dapagliflozin doses higher than 20 mg/day in patients with T2DM [26]. More studies by intravenous routes or in other animal models may provide more evidence confirming or expanding our findings.
3.2 Canagliflozin and Dapagliflozin Pharmacokinetics
The canagliflozin pharmacokinetics in humans show an average bioavailability of 65%. Studies have shown dose proportional Cmax and AUC until 300 mg dose. Its elimination half-life is approximately 10-16 hours. Canagliflozin clearance after intravenous administration was 12.2 L/h, and its volume of distribution was 83.5 L [25, 27]. The pharmacokinetics of dapagliflozin in humans have shown dose-proportional systemic exposure over a wide range between 0.1-500 mg, even though its regular dose is only 10 mg. Its oral bioavailability is 78%, and the volume of distribution is approximately 118 L. The half-life after oral administration is in the range of 10 and 20 h, and the observed oral plasma clearance is 4.9 mL/min/kg [26, 28].
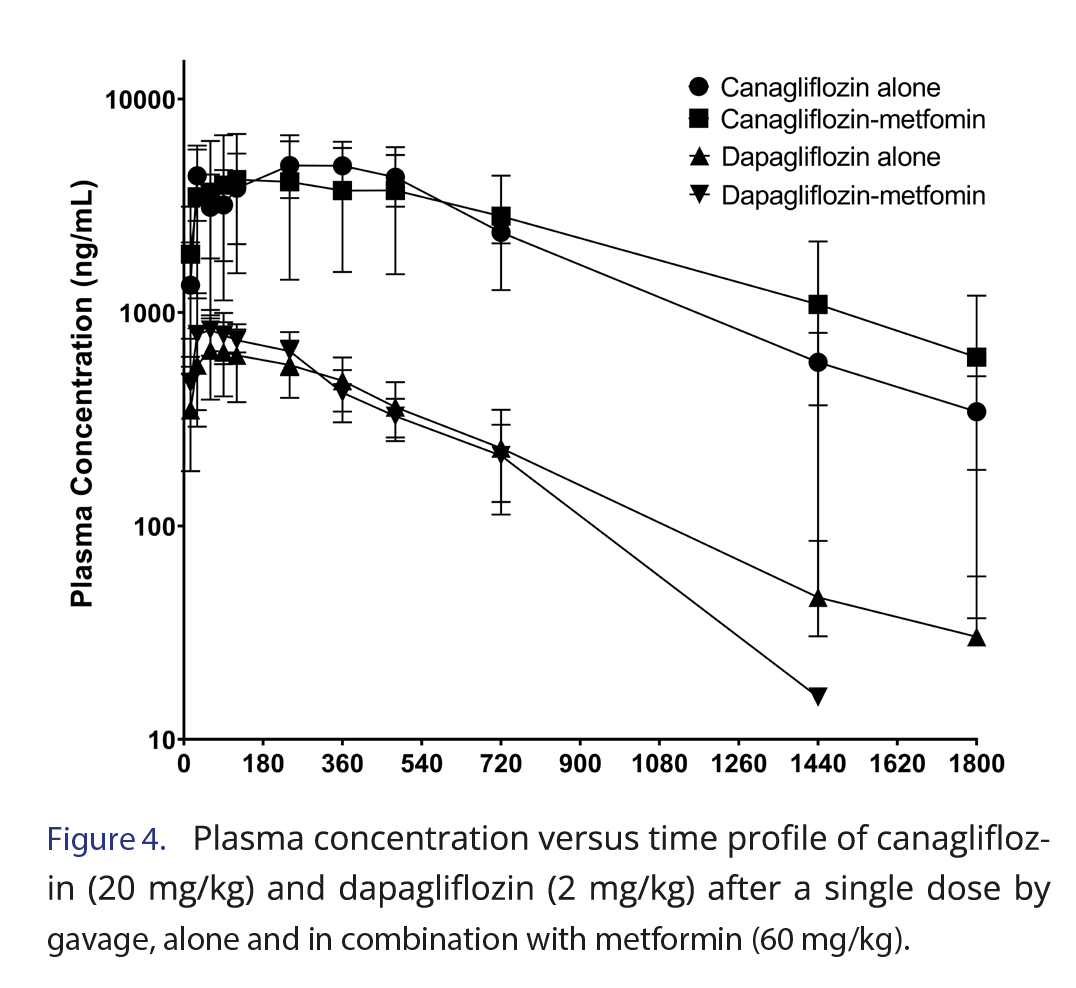
Figure 4 and Table 2 demonstrate the plasma concentration versus time profiles and the pharmacokinetic parameters, respectively, of canagliflozin and dapagliflozin after gavage administration in rats, alone and in combination with metformin. For canagliflozin, the Cmax of 6116.7 ng/mL observed in our study was similar to that observed by Cui and coauthors [29] after dose correction, since they have administered 10 mg/kg. The half-life and volume of distribution were also similar, but the clearance of our study (5.1 mL/min/kg) was higher than that of Cui and coauthors, which was 3.5 mL/min/kg.
Despite small differences, our results were consistent with those in the literature. The study of Obermeier and coauthors [28] by the oral route in rats presented a Cmax of 600 ng/mL after 1 mg/kg, which is double our Cmax of 699.8 ng/mL, since our dose was 2 mg/kg. He and coauthors [30] reported that dapagliflozin CL/F was 3.33 mL/min/kg, while our result was 4.6 mL/min/kg. They also published dapagliflozin half-life and MRT, which were slightly lower than our findings [30].
The plasma concentrations of the SGLT2 inhibitors (canagliflozin or dapagliflozin) alone and in combination with metformin in rats resulted in similar profiles (Figure 4), and no significant difference was observed after parameter comparison between groups alone and metformin (Table 1). Previous studies have shown that SGTL2i drugs are extensively absorbed by the gastrointestinal tract, and their maximum concentrations are observed after 1 or 2 hours in fasted conditions in humans. These drugs undergo hepatic metabolism, mainly by glucuronidation, dealkylation, and oxidation, but they may also be eliminated by excretion in bile or urine [26, 31]. A previous study showed that metformin had no compromising effect on the pharmacokinetic properties of SGLT2 inhibitors drugs [32, 33], which was also observed in our results in a rat model.
4.0 Conclusions
Metformin pharmacokinetics in rats were performed alone and in combination with SGLT2 inhibitors. The overall exposure to metformin was high in the coadministration groups. Canagliflozin and dapagliflozin, two new antihyperglycemic agents from the class of sodium glucose cotransporter 2 inhibitors, had their pharmacokinetics evaluated alone and in the presence of metformin. Both drug pharmacokinetics were not susceptible to changes in the coadministration of metformin.
5.0 Acknowledgements
The authors thank the Ministério da Ciência e Tecnologia (MCT), Ministério da Saúde, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (MCT/CNPq/CT-SAÚDE/MS/SCTIE/DECIT) and Secretaria da Ciência, Tecnologia e Ensino Superior (SETI-PR) for their financial support of the laboratory infrastructure. The authors also thank Lovezutte de Campos, S. G. for providing crucial resources for the writing of this paper.
6.0 Funding
Financial support for this study was provided by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Grant # 435793/2018-7.
7.0 References
- DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, Holst JJ, et al. Type 2 diabetes mellitus. Nat Rev Dis Primers 1(1), 15019 (2015).
https://doi.org/10.1038/nrdp.2015.19
- Mohammed I, Hollenberg MD, Ding H, Triggle CR. A Critical Review of the Evidence That Metformin Is a Putative Anti-Aging Drug That Enhances Healthspan and Extends Lifespan. Front Endocrinol 12, 1-24 (2021).
https://doi.org/10.3389/fendo.2021.718942 - Shurrab NT, Arafa E-SA. Metformin: A review of its therapeutic efficacy and adverse effects. Obes Med 17, 100186. (2020).
https://doi.org/10.1016/j.obmed.2020.100186 - Wang YW, He SJ, Feng X, Cheng J, Luo YT, Tian L, et al. Metformin: a review of its potential indications. Drug Des Devel Ther 11, 2421-2429 (2017).
https://doi.org/10.2147/DDDT.S141675 - American Diabetes A. Standards of Medical Care in Diabetes-2022 Abridged for Primary Care Providers. Clin Diabetes 40(1), 10-38 (2022).
https://doi.org/10.2337/cd22-as01 - Rojas LBA, Gomes MB. Metformin: an old but still the best treatment for type 2 diabetes. Diabetol Metab Syndr 5(1), 1-15 (2013).
https://doi.org/10.1186/1758-5996-5-6 - SBD. Diretriz SBD – diabetes (2022). https://diretriz.diabetes.org.br/. Accessed January 30, 2023.
- Davidson JA, Kuritzky L. Sodium glucose co-transporter 2 inhibitors and their mechanism for improving glycemia in patients with type 2 diabetes. Postgrad Med 126(6), 33-48 (2014).
https://doi.org/10.3810/pgm.2014.10.2819 - Vivian E. Sodium-Glucose Cotransporter 2 Inhibitors in the Treatment of Type 2 Diabetes Mellitus. Diabetes Educ 41(1 Suppl, 5s-18s (2015).
https://doi.org/10.1177/0145721715607643 - Scheen AJ. SGLT2 Inhibitors as Add-On Therapy to Metformin for People with Type 2 Diabetes: A Review of Placebo-Controlled Trials in Asian versus Non-Asian Patients. Diabetes Metab Syndr Obes 13, 2765-2779 (2020).
https://doi.org/10.2147/DMSO.S193528 - Donnan K, Segar L. SGLT2 inhibitors and metformin: Dual antihyperglycemic therapy and the risk of metabolic acidosis in type 2 diabetes. Eur J Pharmacol 846, 23-29 (2019).
https://doi.org/10.1016/j.ejphar.2019.01.002 - Milder TY, Stocker SL, Abdel Shaheed C, McGrath-Cadell L, Samocha-Bonet D, Greenfield JR, et al. Combination Therapy with an SGLT2 Inhibitor as Initial Treatment for Type 2 Diabetes: A Systematic Review and Meta-Analysis. J Clin Med 8(1), 1-14 (2019).
https://doi.org/10.3390/jcm8010045 - Tadesse S, Clinical Pharmacokinetics of Metformin, In Metformin, Juber A, Usama A, Badruddeen, Mohammad Irfan K, (Eds). Metformin. IntechOpen, London United Kingdom, Chapter 3 (2021).
- Heller AA, Lockwood SY, Janes TM, Spence DM. Technologies for Measuring Pharmacokinetic Profiles. Annu Rev Anal Chem 11(1), 79-100 (2018).
https://doi.org/10.1146/annurev-anchem-061417-125611 - de Campos ML, Baldan-Cimatti HM, Davanco MG, Nogueira MA, Padilha EC, Candido CD, et al. Pharmacokinetic profile of a new diclofenac prodrug without gastroulcerogenic effect. Drug Metab Lett 6(4), 235-241 (2012).
https://doi.org/10.2174/1872312811206040002 - Dias BCL, Fachi MM, de Campos ML, Degaut FLD, Peccinini RG, Pontarolo R. A new HPLC-MS/MS method for the simultaneous quantification of SGLT2 inhibitors and metformin in plasma and its application to a pharmacokinetic study in healthy volunteers. Biomed Chromatogr 33(11), 1-12 (2019).
https://doi.org/10.1002/bmc.4663 - U.S. Department of Health and Human Services Food and Drug Administration. Guidance for Industry. Bioanalytical method validation (2018). https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf. Accessed January 30, 2023
- ANVISA. RDC 27 de 17 de maio de 2012: Dispõe sobre os requisitos mínimos para a validação de métodos bioanalíticos empregados em estudos com fins de registro e pós-registro de medicamentos (2012). https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2012/rdc0027_17_05_2012.html. Accessed on January 30, 2023.
- Jingfan Z, Ling L, Cong L, Ping L, Yu C. Efficacy and safety of sodium-glucose cotransporter-2 inhibitors in type 2 diabetes mellitus with inadequate glycemic control on metformin: a meta-analysis. Arch Endocrinol Metab 63(5), 478-486 (2019).
https://doi.org/10.20945/2359-3997000000146 - Molugulu N, Yee LS, Ye YT, Khee TC, Nie LZ, Yee NJ, et al. Systematic review of metformin monotherapy and dual therapy with sodium glucose co-transporter 2 inhibitor (SGLT-2) in treatment of type 2 diabetes mellitus. Diabetes Res Clin Pract 132, 157-168 (2017).
https://doi.org/10.1016/j.diabres.2017.07.025 - Teo YL, Ho HK, Chan A. Metabolism-related pharmacokinetic drug-drug interactions with tyrosine kinase inhibitors: current understanding, challenges and recommendations. Br J Clin Pharmacol 79(2), 241-253 (2015).
https://doi.org/10.1111/bcp.12496 - Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong JK, et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet 50(2), 81-98 (2011).
https://doi.org/10.2165/11534750-000000000-00000 - Choi YH, Kim SG, Lee MG. Dose-independent pharmacokinetics of metformin in rats: Hepatic and gastrointestinal first-pass effects. J Pharm Sci 95(11), 2543-2552 (2006).
https://doi.org/10.1002/jps.20744 - Gong L, Goswami S, Giacomini KM, Altman RB, Klein TE. Metformin pathways: pharmacokinetics and pharmacodynamics. Pharmacogenet Genom 22(11), 820-827 (2012).
https://doi.org/10.1097/FPC.0b013e3283559b22 - Devineni D, Manitpisitkul P, Murphy J, Skee D, Wajs E, Mamidi RN, et al. Effect of canagliflozin on the pharmacokinetics of glyburide, metformin, and simvastatin in healthy participants. Clin Pharmacol Drug Dev 4(3), 226-236 (2015).
https://doi.org/10.1002/cpdd.166 - Kasichayanula S, Liu X, Lacreta F, Griffen SC, Boulton DW. Clinical pharmacokinetics and pharmacodynamics of dapagliflozin, a selective inhibitor of sodium-glucose co-transporter type 2. Clin Pharmacokinet 53(1), 17-27 (2014).
https://doi.org/10.1007/s40262-013-0104-3 - Chen X, Hu P, Vaccaro N, Polidori D, Curtin CR, Stieltjes H, et al. Pharmacokinetics, Pharmacodynamics, and Safety of Single-Dose Canagliflozin in Healthy Chinese Subjects. Clin Ther 37(7), 1483-1492 (2015).
https://doi.org/10.1016/j.clinthera.2015.04.015 - Obermeier M, Yao M, Khanna A, Koplowitz B, Zhu M, Li W, et al. In vitro characterization and pharmacokinetics of dapagliflozin (BMS-512148), a potent sodium-glucose cotransporter type II inhibitor, in animals and humans. Drug Metab Dispos 38(3), 405-414 (2010).
https://doi.org/10.1124/dmd.109.029165 - Cui Y, Li Y, Guo C, Li Y, Ma Y, Dong Z. Pharmacokinetic Interactions between Canagliflozin and Sorafenib or Lenvatinib in Rats. Molecules 27(17), 1-15 (2022).
https://doi.org/10.3390/molecules27175419 - He X, Li Y, Ma Y, Fu Y, Xun X, Cui Y, et al. Development of UPLC-MS/MS Method to Study the Pharmacokinetic Interaction between Sorafenib and Dapagliflozin in Rats. Molecules 27(19), 1-16 (2022).
https://doi.org/10.3390/molecules27196190 - Mamidi RN, Cuyckens F, Chen J, Scheers E, Kalamaridis D, Lin R, et al. Metabolism and excretion of canagliflozin in mice, rats, dogs, and humans. Drug Metab Dispos 42(5), 903-916 (2014).
https://doi.org/10.1124/dmd.113.056440 - Devineni D, Polidori D. Clinical Pharmacokinetic, Pharmacodynamic, and Drug-Drug Interaction Profile of Canagliflozin, a Sodium-Glucose Co-transporter 2 Inhibitor. Clin Pharmacokinet 54(10), 1027-1041 (2015).
https://doi.org/10.1007/s40262-015-0285-z - Kasichayanula S, Liu X, Shyu WC, Zhang W, Pfister M, Griffen SC, et al. Lack of pharmacokinetic interaction between dapagliflozin, a novel sodium-glucose transporter 2 inhibitor, and metformin, pioglitazone, glimepiride or sitagliptin in healthy subjects. Diabetes Obes Metab 13(1), 47-54 (2011).
https://doi.org/10.1111/j.1463-1326.2010.01314.x
All site content, except where otherwise noted, is licensed under a Creative Commons Attribution 4.0 License