OPEN-ACCESS PEER-REVIEWED
RESEARCH ARTICLE
Maria Petrocheilou1, Victoria Samanidou1, Leda Kovatsi2, Magda Tsolaki3, Ioannis Papadoyannis1,*
1Laboratory of Analytical Chemistry, Department of Chemistry, Aristotle University of Thessaloniki, Greece. 2Laboratory of Forensic Medicine and Toxicology, School of Medicine, Aristotle University of Thessaloniki, Greece. 33rd Department of Neurology, GH “G. Papanicolaou”, Aristotle University of Thessaloniki, Greece.
Journal of Applied Bioanalysis. Vol.3. No.4. pages 59-69 (2017)
Published 15 June 2017. https://doi.org/10.17145/jab.17.010 | (ISSN 2405-710X)
Correspondence:
*Ingram J . Department of Chemistry & Biochemistry, Northern Arizona University, P. O. Box 5698, Flagstaff, AZ 86011, USA. Phone: +1-928-523-7877.
Citation:
Jim V, LaViolette C, Briehl MM, Ingram JC. Spatial Distribution of Uranium in Mice Kidneys Detected by Laser Ablation Inductively Coupled Plasma Mass Spectrometry. J Appl Bioanal 3(3), 43-48 (2017).
Open-access and Copyright:
©2017 Jim V et al. This article is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Funding/Manuscript writing assistance:
The authors acknowledge funding from the National Cancer Institute and the Partnership for Native American Cancer Prevention (NAU Grant Number U54CA143925 and UACC Grant Number U54CA143924). Authors decalre that no writing assistance was utilized in the production of this article.
Competing interest:
The authors have declared that no competing interest exists.
Article history:
Received: 27 February 2017, Revised 11 May 2017, Accepted 15 May 2017.
Abstract
Galantamine (GAL), donepezil (DON) and rivastigmine (RIV) are cholinesterase inhibitors administered to patients who suffer from Alzheimer’s disease (AD). We have currently developed and validated an HPLC-DAD method for the determination of GAL, DON, RIV in cerebrospinal fluid (CSF), blood serum and urine. The retention times of the drugs were 1.5, 2.2 and 3.1 min, respectively and the total time of analysis was 5 min. Validation was performed in terms of linearity, selectivity, accuracy, precision and stability. Following validation, the method was applied to clinical CSF, blood serum and urine samples. The currently developed method is a reliable tool for monitoring CSF, serum and urine galantamine, donepezil and rivastigmine levels in patients under treatment with these drugs.
Keywords
Cholinesterase inhibitors, cerebrospinal fluid, blood serum, urine, HPLC, diode array detection.
Introduction
Galantamine, donepezil and rivastigmine are drugs prescribed in Alzheimer’s Disease (AD). Galantamine hydrobromide, donepezil hydrochloride and rivastigmine are inhibitors of acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE), which hydrolyze the neurotransmitter acetylcholine. Therefore, galantamine hydrobromide, donepezil hydrochloride and rivastigmine exert their therapeutic effect by increasing the concentration of acetylcholine and enhancing the cholinergic function [1]. It has been shown that AChE and BuChE inhibition in CSF correlates positively with cognitive performance [2]. Furthermore, the monitoring of the enzyme’s activity and the drug concentration in CSF is of clinical importance according to the latest scientific data[3,4]. Thus, the availability of a reliable analytical technique for the determination of these drugs in CSF, blood serum and urine is of utmost importance both in the clinical, as well as in the forensic toxicology setting.
A number of methods have been developed until today for the separate determination of galantamine, donepezil and rivastigmine in plasma [5-34]. Additionally, a few methods have been reported for the separate determination of these drugs in serum and urine [35-39]. In most of the aforementioned cases, High Pressure Liquid Chromatography, coupled with mass spectrometry or tandem mass spectrometry, was used. UV and fluorescence have also been used for detection. The preparation techniques used were mainly solid phase extraction, liquid-liquid extraction and protein precipitation.
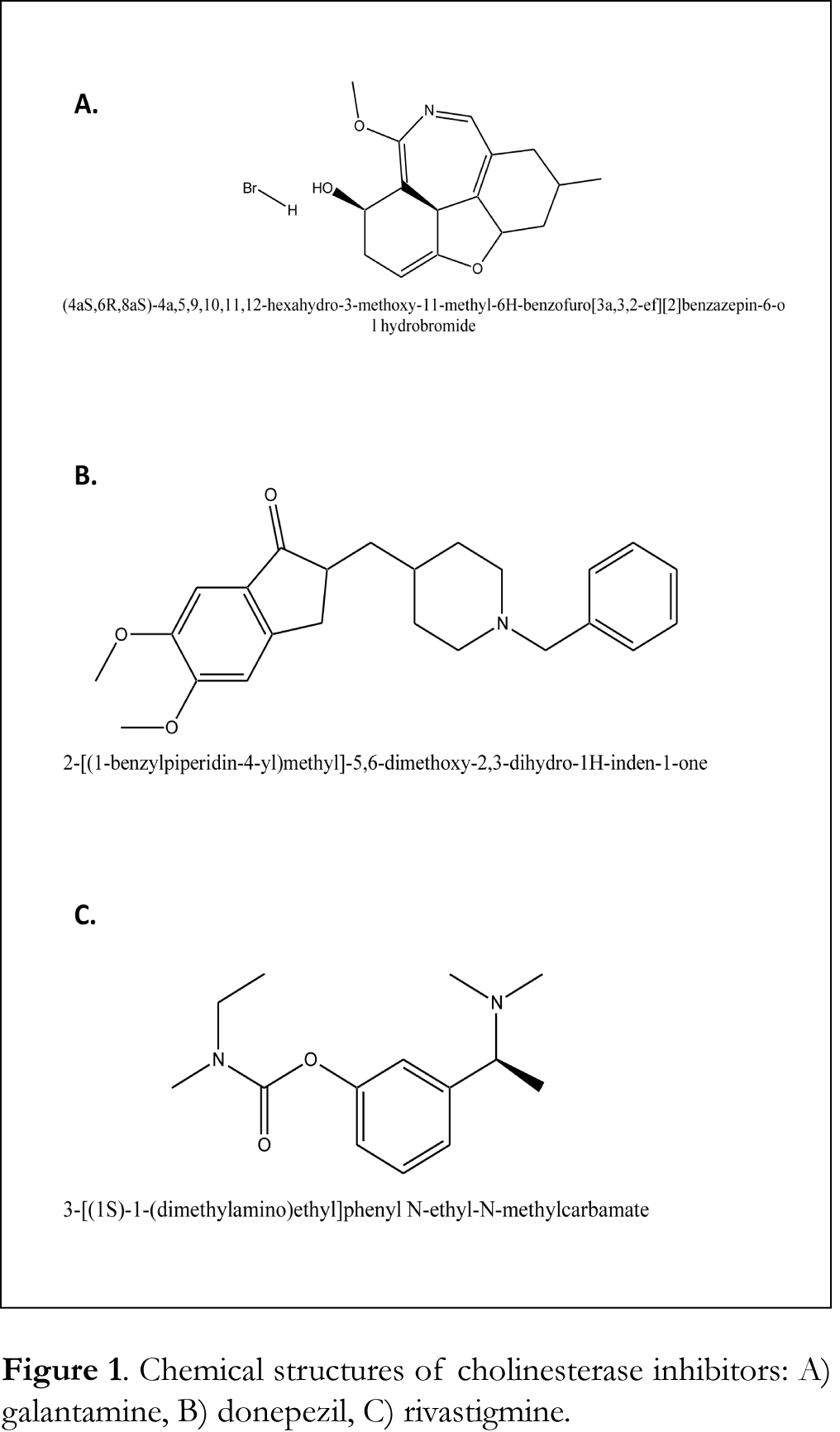
To the best of our knowledge, a fully validated method for the determination of donepezil in CSF has only been reported once until today [40]. Furthermore, only two methods have been developed for the simultaneous detection of four antidementia drugs (donepezil, galantamne, rivastigmine and memantine) in human plasma by LC-MS and UPLC-MS/MS [41,42]. Thus, a validated method for the simultaneous determination of galantamine, donepezil and rivastigmine in CSF, blood serum and urine has not been reported until today. For this reason, we have developed and validated a simple HPLC method, with a use of a diode-array detector, for the determination of these particular AChE and BuChE inhibitors in CSF, blood serum and urine. This method will be further employed into clinical practice in order to assess a possible correlation between the CSF levels of ChE inhibitors, AChE and BuChE activity in CSF and clinical outcome in patients with cognitive impairment. The chemical structures of the drugs of interest used are shown in Figure 1.
Materials and methods
Chemicals and reagents
Acetic acid and sodium acetate trihydrate were purchased from Merck (Darmstadt, Germany). Donepezil hydrochloride was a kind donation from the pharmaceutical company Elpen. Rivastigmine (purity 99,9%) was purchased from the pharmaceutical company Carbosynth (Compton, Berkshire, UK) and galantamine was derived from its pharmaceutical formulation which is commercially available in Greece (Galantamine hydrobromide, 8 mg/capsule, Generics Pharma Hellas, a subsidiary of Mylan INC). All organic solvents (acetonitrile, methanol), as well as water, were of HPLC grade. Acetonitrile was purchased from LAB-SCAN, Analytical Sciences (POCH S.A., Gliwice, Poland). Methanol was purchased from Chromasolv (Fluka Analytical, Sigma-Aldrich, USA). Water was purchased from Chromasolv®Plus (Sigma-Aldrich, USA). Ammonia was purchased from Panreac Quimica S.A. (Barcelona, Spain). Colchicine, tolfenamic acid, chlortetracycline, chloramphenicol and thiamphenicol were purchased from Sigma-Aldrich (PA, USA). Lamotrigine, sulfamethazine, sulfisoxazole, sulfadiazine, sulfaquinoxaline and sulfamethizole were purchased from Sigma-Aldrich (Saint-Louis, MO, USA). Nalidixic acid and flumequine were purchased from Sigma (Steinheim, Germany). Oxolinic acid was purchased from Fluka (Buchs, Switzerland). Epichlorotetracycline was purchased from Fisher Scientific (Germany). Bamifyline was purchased from LGM Pharma, USA. Doxycycline was purchased from Oracea (USA). The above compounds were all evaluated as possible internal standards.
Preparation of standards
Stock standard solutions of the examined drugs (160 ng/μL for galantamine, 1000 ng/μL for donepezil and rivastigmine) were prepared in methanol, with the exception of donepezil, for which the stock standard solution was prepared in water, due to the better solubility of the drug in this solvent. All standard solutions were stored at 4°C. From the above stock standard solutions, eight aquatic working standards were prepared in the range of 0.5-40 ng/μL. Calibration standards of the cholinergic drugs in the three different biological matrices were prepared by spiking drug-free samples with standard solutions.
Instrumentation and Chromatography
Analysis was performed on an UHPLC system which consisted of a Shimadzu Prominence liquid chromatograph with a 10 μL injection loop, coupled with a Shimadzu SPD-M20A Diode Array UV detector (Kyoto, Japan). The analysis was carried out on an ODS Hypersil column (C18 classical, 250×2,1 mm, 5 μm) (Thermo Fisher Scientific, Germany). The mobile phase consisted of Solvent A: acetonitrile, Solvent B: methanol and Solvent C: buffer solution of sodium acetate/acetic acid, 0.2M, pH 4.8. The time program used was: 0 min-solvent A 45% (v/v), solvent B 20% (v/v) and solvent C 35% (v/v) – flow rate at 0.6 mL/min, 3 min-solvent A 45% (v/v), solvent B 45% (v/v) and solvent C 10% (v/v) – flow rate at 0.9 mL/min. The maximum inlet pressure was 3800 psi. Analysis was completed within 5 min. UV detection was performed at 215 nm (rivastigmine), 232 nm (donepezil) and 290 nm (galantamine). Data acquisition was performed by the LCsolution, version 1.25 (Shimadzu Corporation) software.
Sample collection
Post-mortem, drug-free CSF samples were collected, as part of the standard operating procedure, upon autopsy at the Forensic Medical Service of Thessaloniki, Greece. Blood serum and urine were collected from healthy volunteers, who refrained from using any medication. Clinical CSF, blood and urine samples were collected from patients under treatment with cholinergic drugs, following written informed consent.
Sample preparation
The CSF samples (50 μL) were subjected to protein precipitation with the addition of 100 μL of CH3CN. Following centrifugation, the supernatant was evaporated (not to dryness) under a gentle stream of nitrogen and was then loaded onto the pre-conditioned SPE cartridge. The blood serum samples (50 μL) were subjected to protein precipitation with the addition of 400 μL CH3CN. Following centrifugation, the supernatant was evaporated (not to dryness) under a gentle stream of nitrogen and was then loaded onto the pre-conditioned SPE cartridge. The urine samples (50 μL) were treated in the same way as the CSF samples and were then loaded onto the pre-conditioned SPE cartridge.
Solid phase extraction procedure
For all biological matrices, the cartridges were initially conditioned with 2 mL CH3OH and equilibrated with 2 mL H2O. The sample was then loaded onto the cartridge StrataTM-X-Drug B 33 μm Polymeric Strong Cation [30 mg/3 mL, Phenomenex® (USA)]. The cartridge was washed with 500 μL H2O and the drugs were eluted with 1 mL CH3OH (containing 5% ammonia). The eluent was evaporated to dryness under a gentle stream of nitrogen. It was then reconstituted with 200 μL of CH3OH and 10 μL were injected onto the HPLC column.
Method Validation
Linearity
Linear regression analysis was used to determine linearity. For this purpose, working standard solutions and spiked CSF, blood serum and urine samples, containing cholinergic drugs at different concentrations, within the range of 0.5-40 ng/μL for donepezil and galantamine and 5-40 ng/μL for rivastigmine, were used. The peak area ratio of the analyte was plotted versus the nominal drug concentration in order to construct the calibration curve. Least square linear regression analysis was used to calculate the slope, intercept, and coefficient of determination. The formula LOD= 3.3 S/N was used for the calculation of the limit of detection (LOD) and the formula LOQ= 10 S/N was used for the calculation of the limit of quantitation (LOQ), where S= signal, and N= noise.
Recovery and Precision/Accuracy
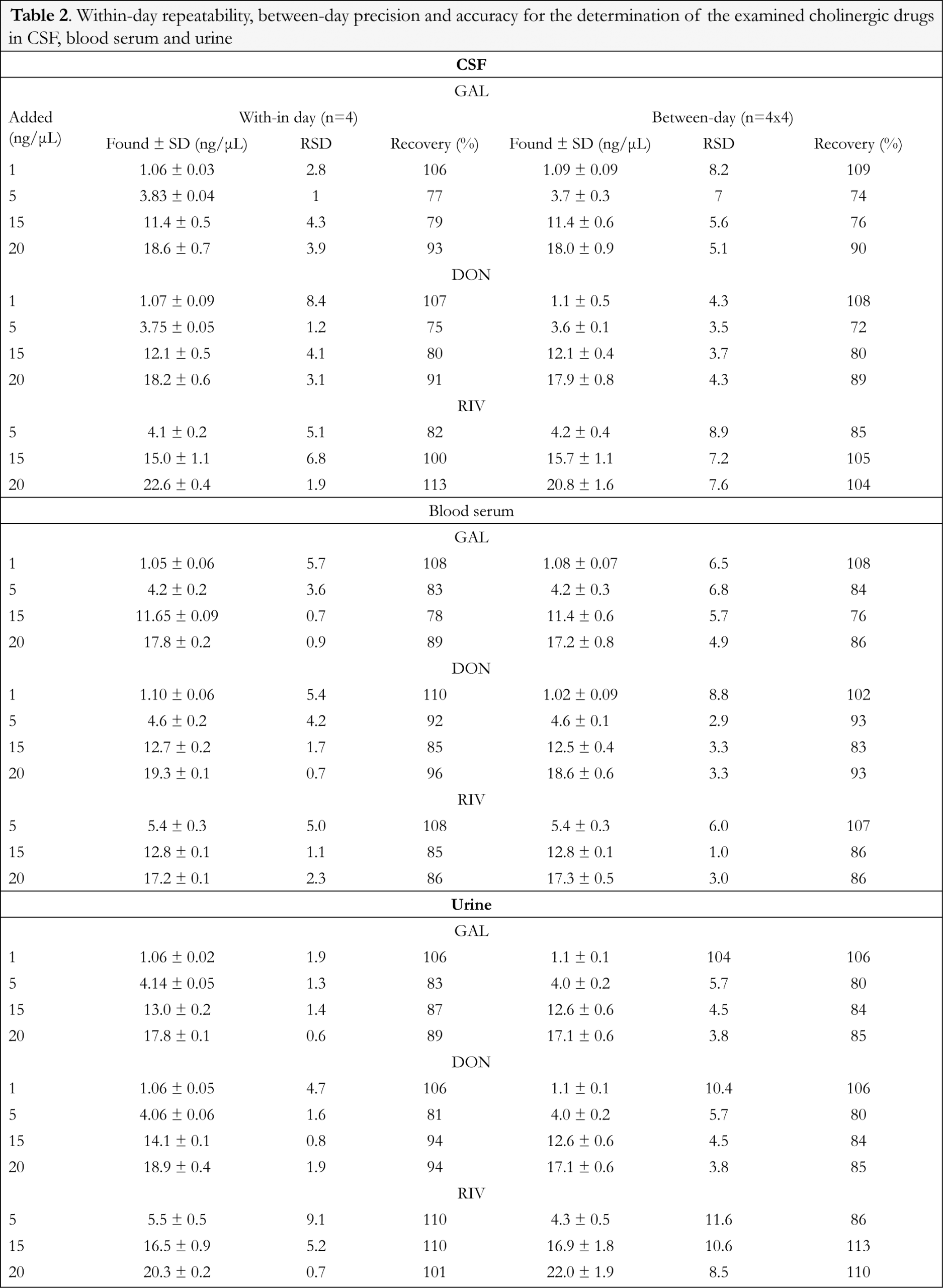
Mean absolute recovery of galantamine was evaluated by comparing spiked CSF, blood serum and urine samples to standard solutions. It was found to be 96%, 85% and 96%, respectively in the three biological matrices. Similarly, mean absolute recovery of donepezil was found to be 96% in CSF, 99% in blood serum and 97% in urine. Mean absolute recovery of rivastigmine was found to be 86% in CSF, 76% in blood serum and 81% in urine. In order to evaluate the precision and accuracy of the developed method, CSF, blood serum and urine samples, spiked at different concentration levels (in the range 0.5-40 ng/μL for donepezil and galantamine and 5-40 ng/μL for rivastigmine), were analyzed. The respective linear regression equation was always used to calculate the nominal contents of the drug. The results were expressed as percent recoveries (mean found concentration/theoretical concentration)/100. The within-day RSD was used as a measure of the repeatability of the method, while the SD between nominal and measured concentrations was used to express precision. CSF, blood serum and urine samples spiked at four different concentrations were analyzed multiple times in order to determine within-day and between-day precision and accuracy. Four measurements performed on the same day were used to assess within-day repeatability. Furthermore, four measurements at four consecutive days were used to evaluate between-day repeatability.
Selectivity
The selectivity of the developed method was demonstrated by the absence of interference in the chromatographic window of interest from degradation products and endogenous matrix components. The selectivity of the method was evaluated by analyzing drug-free CSF, blood serum and urine samples.
Stability
As part of the validation process, we evaluated both the long- and the short- term stability of galantamine, donepezil and rivastigmine in CSF, blood serum and urine. For this purpose, drug-free CSF, blood serum and urine samples were spiked with the three drugs at a final concentration of 15 ng/μL. The stability of cholinergic drugs was assessed under different storage conditions (at 25°C for 1, 2 and 24 h, at 4°C for 1, 2, 3 days, and at -24°C for 1, 2, 4, 6 weeks). The stability of galantamine, donepezil and rivastigmine was also evaluated during the repetitive freezing and thawing of CSF, blood serum and urine. For this purpose, the 10% degradation criterion was used to evaluate stability.
Results and discussion
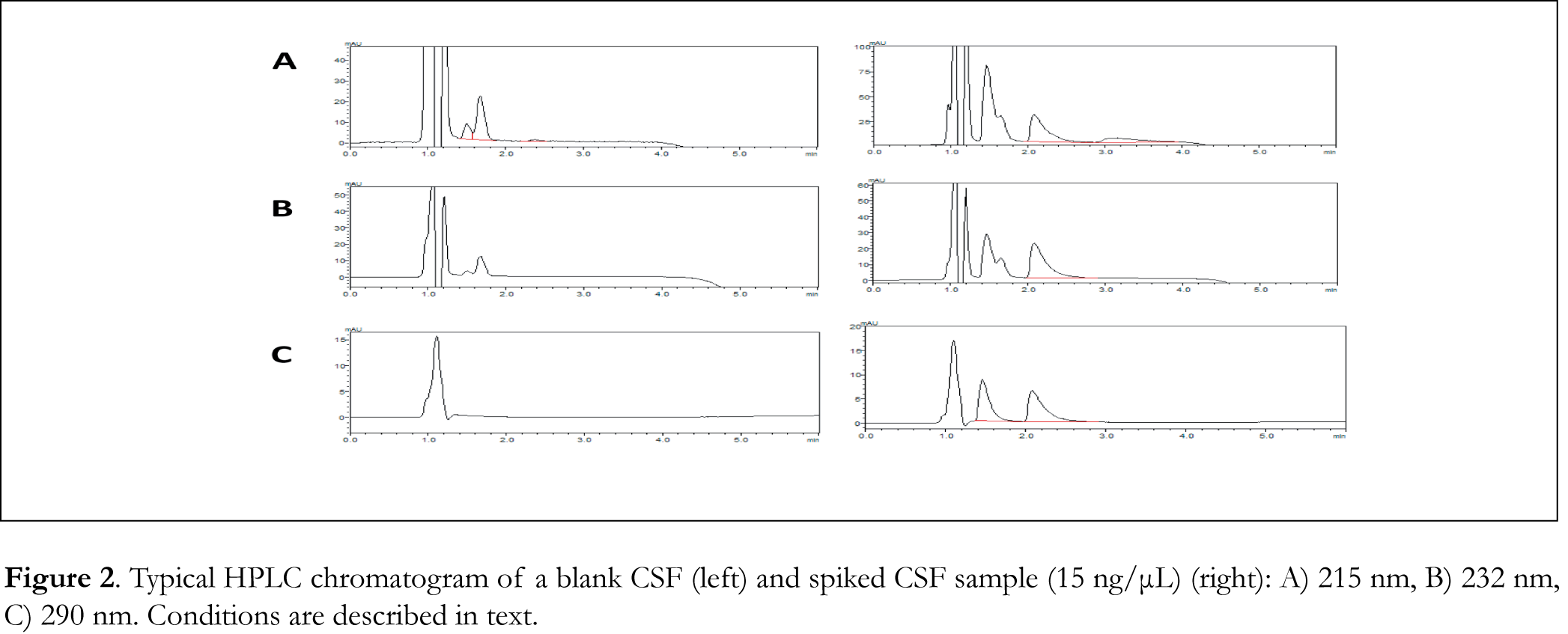
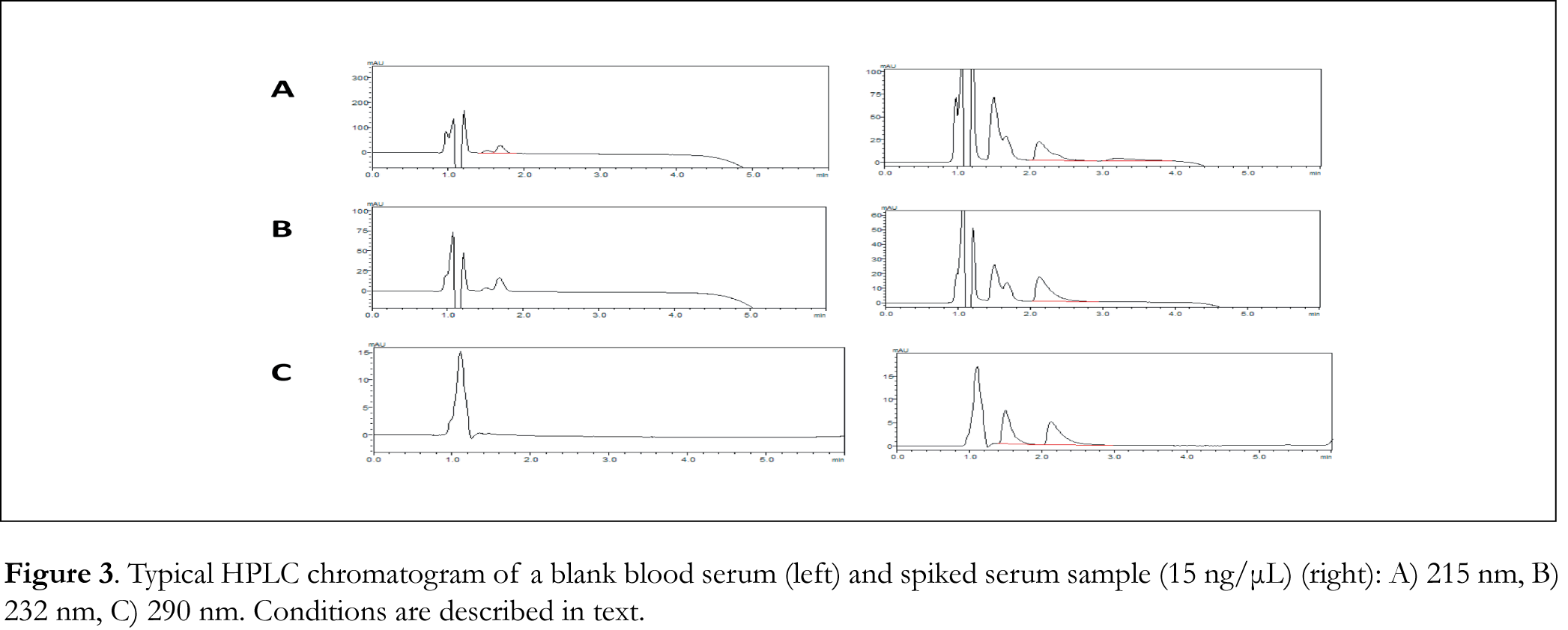
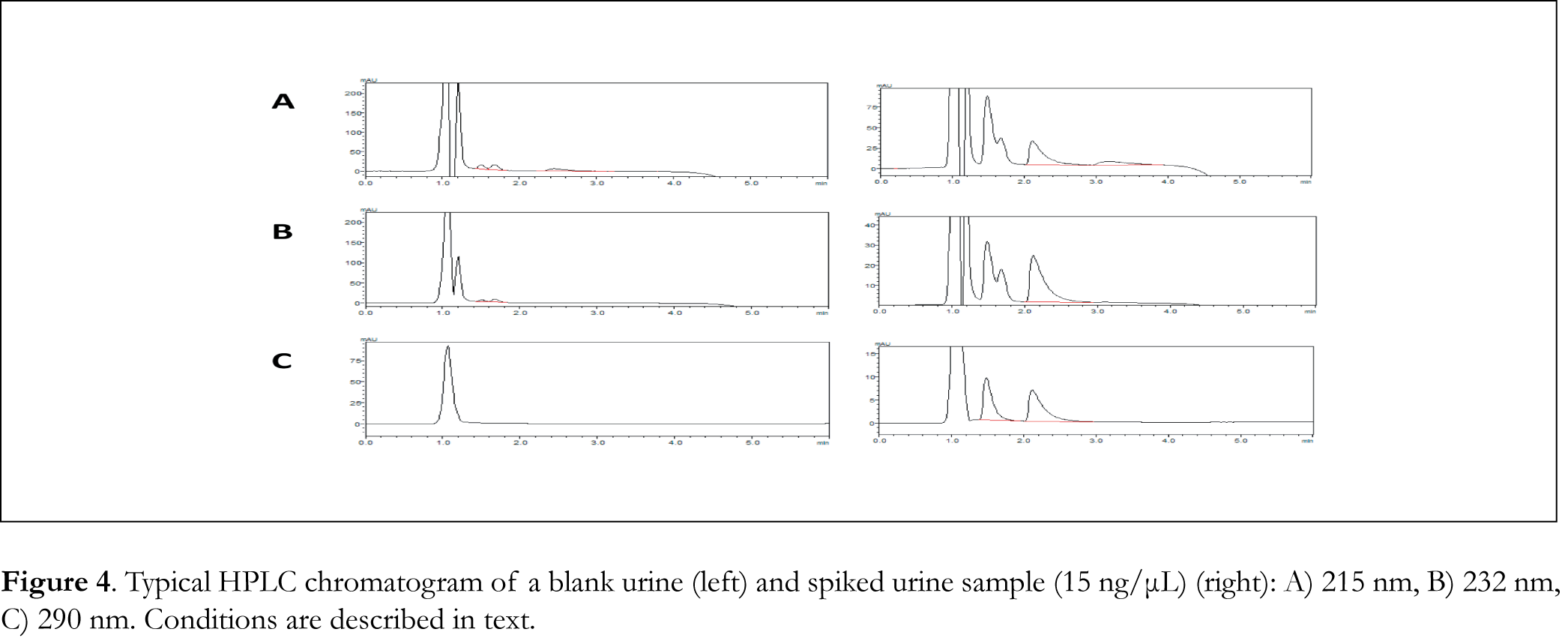
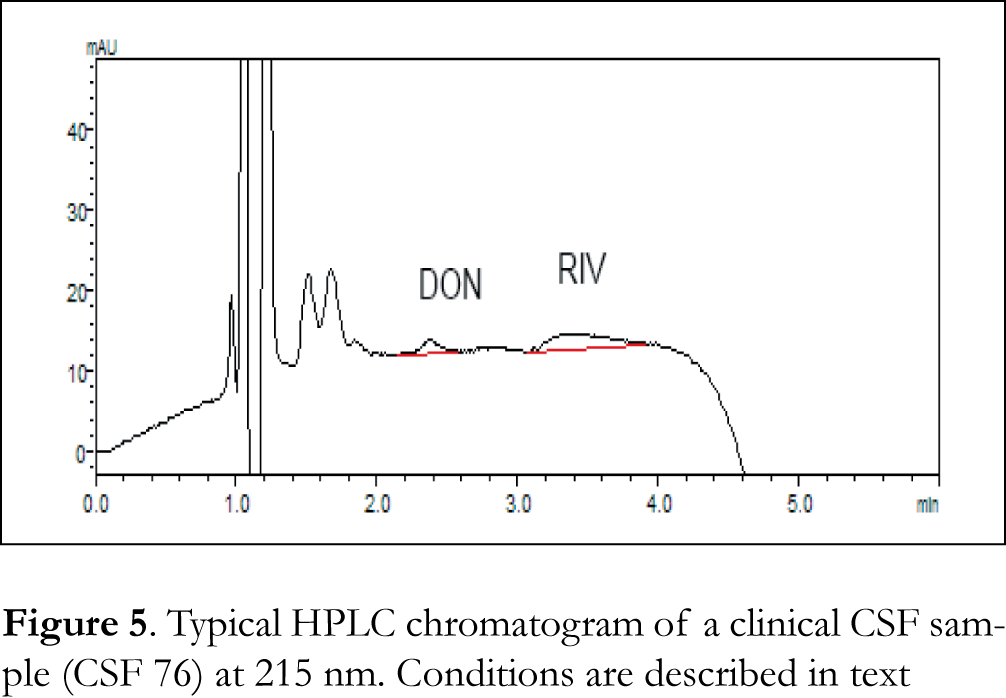
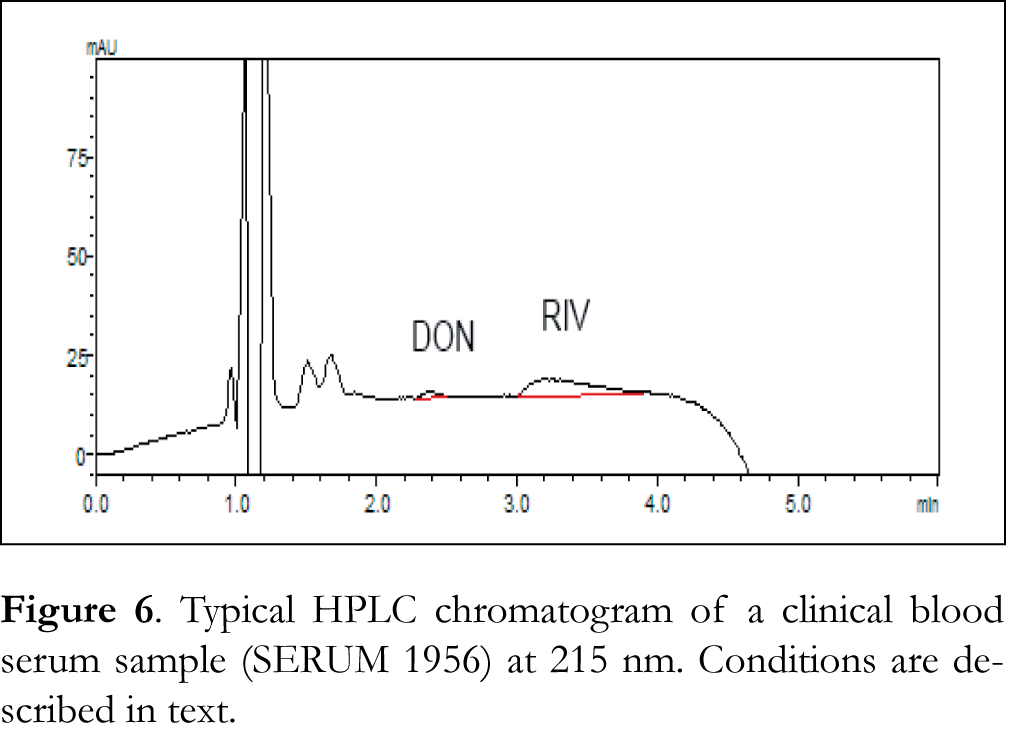
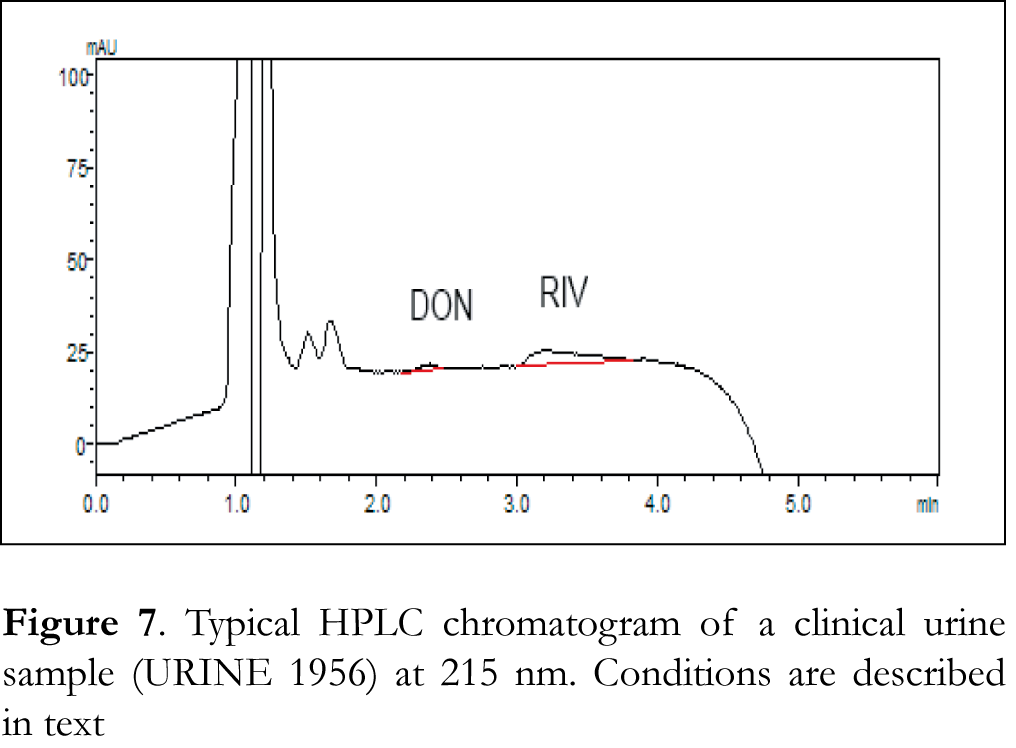
Typical chromatograms of drug-free (blank) CSF, blood serum and urine samples as well as typical chromatograms of spiked CSF, blood serum and urine samples are shown in Figures 2, 3 and 4 respectively. The retention time for galantamine, donepezil and rivastigmine was 1.5, 2.2 and 3.1 min, respectively. The linearity was evaluated in the 0.5-40 ng/μL range for galantamine and donepezil and 5-40 ng/μL for rivastigmine. Linear-regression analysis was applied. Linearity and sensitivity data are summarized in Table 1. The accuracy (assessed by means of recovery percentage), was evaluated by the determination of galantamine, donepezil and rivastigmine in spiked CSF, blood serum and urine samples at four concentration levels. Repeatability was evaluated by analyzing samples spiked with the analyte at four concentration levels, in four replicates. Intermediate precision was assessed by the between-day variation over a period of four days. Accuracy and precision data are summarized in Table 2. Selectivity (absence of interference from the sample matrix in the same chromatographic window as the examined drugs) was evaluated by the analysis of blank CSF, blood serum and urine samples. As shown in Figure 2 (blank CSF sample), Figure 3 (blank blood serum sample) and Figure 4 (blank urine sample) there was no interference in the analysis of the selected drugs from the particular biological matrices examined. To examine the stability of the analytes, drug-free CSF, blood serum and urine samples were spiked with galantamine, donepezil and rivastigmine at a final concentration of 15 ng/μL. Stability was assessed following storage at ambient temperature (25°C) for 1, 2 and 24 h, at 4°C for 1, 2 and 3 days, and at -24°C for 1, 2, 4 and 6 weeks.
During storage of spiked CSF samples at -24°C, galantamine remained stable for 4 weeks, donepezil for 2 weeks and rivastigmine for 1 week. During storage at 4°C, galantamine and donepezil remained stable for 3 days, while rivastigmine remained stable only for 1 day. During storage at ambient temperature (25°C) all three drugs remained stable for 2 hours (h). To examine the stability of galantamine, donepezil and rivastigmine in CSF during repetitive freeze-thaw cycles (from -24°C to room temperature), drug-free CSF was spiked at a final concentration of 15 ng/μL for galantamine, donepezil and rivastigmine. In CSF, galantamine remained stable for 2 cycles, donepezil for 4 cycles and rivastigmine for 1 cycle. Degradation was evaluated using the 10% criterion.
During storage of spiked blood serum samples at -24°C, all three drugs remained stable for 4 weeks. During storage at 4°C, all three drugs remained stable for 3 days. During storage at ambient temperature (25°C) all three drugs remained stable for 2 hours (h). To examine the stability of galantamine, donepezil and rivastigmine in blood serum during repetitive freeze-thaw cycles (from -24°C to room temperature), drug-free blood serum was spiked at a final concentration of 15 ng/μL for galantamine, donepezil and rivastigmine. In blood serum, all three drugs remained stable for 1 cycle. Degradation was evaluated using the 10% criterion. During storage of spiked urine samples at -24°C, all three drugs remained stable for 4 weeks. During storage at 4°C, donepezil and galantamine remained stable for 3 days, while rivastigmine remained stable for 1 day. During storage at ambient temperature (25°C) galantamine and donepezil remained stable for 2 hours (h), while rivastigmine remained stable only for 1 hour. To examine the stability of galantamine, donepezil and rivastigmine in urine during repetitive freeze-thaw cycles (from -24°C to room temperature), drug-free urine was spiked at a final concentration of 15 ng/μL for galantamine, donepezil and rivastigmine. In urine, galantamine remained stable for 2 cycles, donepezil for 6 cycles and rivastigmine for 4 cycles. Degradation was evaluated using the 10% criterion.
Figures and Tables
[Click to enlarge]
Application of the developed method to clinical CSF, blood serum and urine samples
The developed method was applied to clinical CSF, blood serum and urine samples obtained from patients under treatment with donepezil and rivastigmine. Typical chromatograms of clinical CSF, blood serum and urine samples are shown in Figures 5, 6 and 7, respectively. The standard addition method was applied to some samples where donepezil and rivastigmine concentration was below the LLOQ of the method. Table 3 shows the concentrations of donepezil and rivastigmine in the different clinical CSF, blood serum and urine samples analyzed.
Conclusions
The degree of AChE and BuChE inhibition in CSF, by AChE and BuChE inhibitors (such as donepezil, galantamine and rivastigmine) is of clinical importance in patients with cognitive impairment and is possibly associated with the concentration of the inhibitor in CSF. There are currently two validated methods for the simultaneous determination of galantamine, donepezil and rivastigmine by HPLC-MS/MS and UHPLC-MS/MS, respectively. Because mass detection demands expensive instrumentation, we have currently developed and validated a fast and simple HPLC-DAD method for the simultaneous determination of three cholinergic drugs in CSF, blood serum and urine. The proposed method was validated in terms of linearity, selectivity, accuracy, precision and stability and proved to be robust and reliable. The developed method will be further employed in order to assess a possible correlation between the CSF levels of ChE inhibitors, AChE and BuChE activity in CSF and clinical outcome in patients with cognitive impairment. This method can also be applied in the clinical toxicology setting, as well as in the forensic toxicology setting and to the best of our knowledge, it is the only available method for the simultaneous determination of these drugs in CSF, urine and blood serum by a simple HPLC-DAD method.
Acknowledgement
The authors wish to thank ELPEN Pharmaceutical Co. Inc., Pikermi Attica, Greece, for kind donation of donepezil and the company Chemic. S-Phenomenex for granting the SPE cartridges Strata X Drug-B Strong Cation.
References
1. Ponnayyan Sulochana S, Sharma K, Mullangi R et al. Review of the validated HPLC and LC-MS/MS methods for determination of drugs used in clinical practice for Alzheimer’s disease. Biomed Chromatogr 28 (11), 1431-1490 (2014). [CrossRef]
2. Darreh-Shori T, Meurling L, Pettersson T et al. Changes in the Activity and Protein Levels of CSF Acetylcholinesterases in Relation to Cognitive Function of Patients with Mild Alzheimer’s Disease Following Chronic Donepezil Treatment. J Neural Transm 113, 1791–1801 (2006). [CrossRef]
3. Parnetti L, Amici S, Lanari A et al. Cerebrospinal Fluid Levels of Biomarkers and Activity of Acetylcholinesterase (AChE) and Butyrylcholinesterase in AD Patients Before and After Treatment with Different AChE Inhibitors. Neurol Sci 23, 895–896 (2002). [CrossRef]
4. Parnetti L, Chiasserini D, Andreasson U et al. Changes in CSF Acetyl- and Butyrylcholinesterase Activity After Long-term Treatment with AChE Inhibitors in Alzheimer’s Disease. Acta Neurol Scand 124, 122–129 (2011). [CrossRef]
5. Abonassif MA, Hefnawy MM, Kassem MG et al. Determination of donepezil hydrochloride in human plasma and pharmaceutical formulations by HPLC with fluorescence detection. Acta Pharmaceut 61, 403–413 (2011). [CrossRef]
6. Radwan MA, Abdine HH, Al-Quadeb BT et al. Stereoselective HPLC assay of donepezil enantiomers with UV detection and its application to pharmacokinetics in rats. J Chromatogr B 830, 114–119 (2006). [CrossRef]
7. Nakashima K, Itoh K, Kono M et al. Determination of donepezil hydrochloride in human and rat plasma, blood and brain microdialysates by HPLC with a short C30 column. J Pharmaceut Biomed 41, 201–206 (2006). [CrossRef]
8. Yasui-Furukori N, Furuya R, Takahat T et al. Determination of donepezil, an acetylcholinesterase inhibitor, in human plasma by high-performance liquid chromatography with ultraviolet absorbance detection. J Chromatogr B 768, 261–265 (2002). [CrossRef]
9. Iordachescu A, Silvestro L, Tudoroniu A et al. LC-MS-MS method for the simultaneous determination of donepezil enantiomers in plasma. Chromatographia 75, 857–866 (2012) . [CrossRef]
10. Abonassif MA, Hefnawy MM, Kassem MG et al. Determination of donepezil hydrochloride in human plasma and pharmaceutical formulations by HPLC with fluorescence detection. Acta Pharmaceut 61, 403–413 (2011). [CrossRef]
11. Khuroo AH, Gurule SJ, Monif T et al. ESI-MS/ MS stability-indicating bioanalytical method development and validation for simultaneous estimation of donepezil, 5-desmethyl donepezil and 6-desmethyl donepezil in human plasma. Biomed Chromatogr 26, 636–649 (2012). [CrossRef]
12. Kim KA, Lim JL, Kim C et al. Pharmacokinetic comparison of orally disintegrating and conventional donepezil formulations in healthy Korean male subjects: a single-dose, randomized, open-label, 2- sequence, 2-period crossover study. Clin Ther 33, 965–972 (2011). [CrossRef]
13. Pilli NR, Inamadugu JK, Kondareddy N et al. A rapid and sensitive LC-MS/MS method for quantification of donepezil and its active metabolite, 6-O-desmethyl donepezil in human plasma and its pharmacokinetic application. Biomed Chromatogr 25, 943–951 (2011). [CrossRef]
14. Shah HJ, Kundlik ML, Pandya A et al. A rapid and specific approach for direct measurement of donepezil concentration in human plasma by LC-MS/MS employing solid-phase extraction. Biomed Chromatogr 23, 141–151 (2009). [CrossRef]
15. Patel BN, Sharma N, Sanyal M et al. Quantitation of donepezil and its active metabolite 6-O-desmethyl donepezil in human plasma by a selective and sensitive liquid chromatography–tandem mass spectrometric method. Anal Chim Acta 629, 145–157 (2008). [CrossRef]
16. Park EJ, Lee HW, Ji HY et al. Hydrophilic interaction chromatography–tandem mass spectrometry of donepezil in human plasma: application to a pharmacokinetic study of donepezil in volunteers. Archives in Pharmaceutical Research 31, 1205–1211 (2008). [CrossRef]
17. Apostolou C, Dotsikas Y, Kousoulos C et al. Quantitative determination of donepezil in human plasma by liquid chromatography tandem mass spectrometry employing an automated liquid–liquid extraction based on 96-well format plates application to a bioequivalence study. J Chromatogr B 848, 239–244 (2007). [CrossRef]
18. Xie Z, Liao Q, Xu X et al. Rapid and sensitive determination of donepezil in human plasma by liquid chromatography/tandem mass spectrometry: application to a pharmacokinetic study. Rapid Commun Mass Sp 20, 3193–3198 (2006). [CrossRef]
19. Lu Y, Wen H, Li W et al. Determination of donepezil hydrochloride (E2020) in plasma by liquid chromatography mass spectrometry and its application to pharmacokinetic studies in healthy, young, Chinese subjects. J Chromatogr Sci 42, 234–237 (2004). [CrossRef]
20. Matsui K, Oda Y, Nakata H et al. Simultaneous determination of donepezil (Aricept) enantiomers in human plasma by liquid chromatography–electrospray tandem mass spectrometry. J Chromatogr B 729, 147–155 (1999). [CrossRef]
21. Matsui K, Oda Y, Ohe H et al. Direct determination of E2020 enantiomers in plasma by liquid chromatography–mass spectrometry and column-switching techniques. J Chromatogr A 694, 209–218 (1995). [CrossRef]
22. Zhang LJ, Fang XL, Li XN et al. Pharmacokinetics and bioequivalence studies of galantamine hydrobromide dispersible tablet in healthy male Chinese volunteers. Drug Dev Ind Pharm 33, 335–340 (2007). [CrossRef]
23. Park YS, Kim SH, Kim SY et al. Quantification of galantamine in human plasma by validated liquid chromatography-tandem mass spectrometry using glimepiride as an internal standard: application to bioavailability studies in 32 healthy Korean subjects. J Chromatogr Sci 50, 803–809 (2012).
24. Steiner WE, Pikalov IA, Williams PT et al. Development of a Novel LC/MS/MS extraction assay for galanthamine in guinea pig plasma and its application to nerve agent countermeasures. Sci Rep 1, 149 (2012). [CrossRef]
25. Malakova J, Nobilis M, Svoboda Z et al. High performance liquid chromatographic method with UV photodiode array, fluorescence and mass spectrometric detection for simultaneous determination of galantamine and its phase I metabolites in biological samples. J Chromatogr B 853, 265–274 (2007). [CrossRef]
26. Nirogi RV, Kandikere VN, Mudigonda K et al. Quantitative determination of galantamine in human plasma by sensitive liquid chromatography–tandem mass spectrometry using loratadine as an internal standard. J Chromatogr Sci 45, 97–103 (2007). [CrossRef]
27. Verhaeghe T, Diels L, de Vries R et al. Development and validation of a liquid chromatographic–tandem mass spectrometricmethod for the determination of galantamine in human heparinised plasma. J Chromatogr B 789, 337–346 (2003). [CrossRef]
28. Arumugam K, Chamallamudi M, Mallayasamy S et al. High performance liquid chromatographic fluorescence detection method for the quantification of rivastigmine in rat plasma and brain: application to preclinical pharmacokinetic studies in rats. J Young Pharm 3, 315–321 (2011). [CrossRef]
29. Amini H and Ahmadiani A. High performance liquid chromatographic determination of rivastigmine in human plasma for application in pharmacokinetic studies. J Pharm Res 9, 115–121 (2010).
30. Karthik A, Subramanian GS, Surulivelrajan M et al. Fluorimetric determination of rivastigmine in rat plasma by a reverse phase high performance liquid chromatographic method. Application to a pharmacokinetic study. Arzneimittelforschung 58, 205–210 (2008).
31. Bhatt J, Subbaiah G, Kambli S et al. A rapid and sensitive liquid chromatography tandem mass spectrometry (LC-MS/MS) method for the estimation of rivastigmine in human plasma. J Chromatogr B 852, 115–121 (2007). [CrossRef]
32. Frankfort SV, Ouwehand M, van Maanen MJ et al. A simple and sensitive assay for the quantitative analysis of rivastigmine and its metabolite NAP 226-90 in human EDTA plasma using coupled liquid chromatography and tandem mass spectrometry. Rapid Commun Mass Sp 20, 3330–3336 (2006). [CrossRef]
33. Enz A, Chappuis A and Dattler A. A simple, rapid and sensitive method for simultaneous determination of rivastigmine and its major metabolite NAP 226-90 in rat brain and plasma by reversed-phase liquid chromatography coupled to electrospray ionization mass spectrometry. Biomed Chromatogr 18, 160–166 (2004). [CrossRef]
34. Pommier F and Frigola R. Quantitative determination of rivastigmine and its major metabolite in human plasma by liquid chromatography with atmospheric pressure chemical ionization tandem mass spectrometry. J Chromatogr B 784, 301–313 (2003). [CrossRef]
35. Koeber R, Kluenemann HH, Waimer R et al. Ιmplementation of a cost-effective HPLC/UV-approach for medical routine quantification of donepezil in human serum. J Chromatogr B Analyt Technol Biomed Life Sci 881-882, 1-11 (2012).
36. Claessens HA, Van Thiel M, Westra P et al. High-performance liquid chromatographic determination of galanthamine, a long-acting anticholinesterase drug, in serum, urine and bile. J Chromatogr 275(2), 345-353 (1983). [CrossRef]
37. Thevis M, Wilkens F, Geyer H et al. Determination of therapeutics with growth hormone secretagogue activity in human urine for doping control purposes. Rapid Commun Mass Sp 20, 3393–3402 (2006). [CrossRef]
38. Arumugam K, Chamallamudi MR, Gilibili RR et al. Development and validation of a HPLC method for quantification of rivastigmine in rat urine and identification of novel metabolite in urine by LC-MS/MS. Biomed Chromatogr 25, 353–361 (2011). [CrossRef]
39. Tencheva J, Yamboliev I and Zhivkova Z. Reversed-phase liquid chromatography for the determination of galanthamine and its metabolites in human plasma and urine. J Chromatogr B 21, 396–400 (1987). [CrossRef]
40. Kovatsi L, Tsolaki M, Gkatzima O et al. Simple UHPLC-DAD Method for the Direct Determination of Donepezil in Cerebrospinal Fluid. J Liq Chromatogr R T 38, 1068–1072 (2015). [CrossRef]
41. Noetzli M, Choong E, Ansermot N et al. Simultaneous determination of antidementia drugs in human plasma for therapeutic drug monitoring. Ther Drug Monit 33, 227–238 (2011). [CrossRef]
42. Noetzli M, Ansermot N, Dobrinas M et al. Simultaneous determination of antidementia drugs in human plasma: procedure transfer from HPLC-MS to UPLC–MS/MS. J Pharmaceut Biomed 64–65, 16–25 (2012). [CrossRef]
All site content, except where otherwise noted, is licensed under a Creative Commons Attribution 4.0 License