OPEN-ACCESS PEER-REVIEWED
EXPERT OPINION
Basant Giri
Center for Analytical Sciences, Kathmandu Institute of Applied Sciences, Kathmandu, Nepal.
Journal of Applied Bioanalysis. Vol.2. No.1. pages 6-9 (2016)
Published 15 January 2016. https://doi.org/10.17145/jab.16.002 | (ISSN 2405-710X)
Correspondence:
Giri B . Center for Analytical Sciences, Kathmandu Institute of Applied Sciences, PO Box 23002, Kathmandu, Nepal. Phone/fax: + 977 01 6924204.
Citation:
Giri B. A perspective on the sensitivity of paper-analytical devices for bioanalysis. J Appl Bioanal 2(1), 6-9 (2016).
Open-access and Copyright:
©2016 Giri B. This article is an open access article distributed under the terms of the Creative Commons Attribution License (CC-BY) which permits any use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Funding/Manuscript writing assistance:
The author has no financial support or funding to report and also declares that no writing assistance was utilized in the production of this article.
Competing interest:
The author has declared that no competing interest exist.
Article history:
Received: 11 December 2015, Revised 22 December 2015, Accepted 23 December 2015.
Keywords
µPADs, signal amplification, limit of detection, disease diagnosis, isotachophoresis on paper
Introduction
Paper-based microfluidic analytical devices (µPADs) have emerged as an attractive platform for chemical and biological sensing in past decade. Paper is made up of cellulose, which is hydrophilic and a permeable porous material consisting of microfibers of 1–100 μm diameter and pore size of 1–10 μm. Some characteristic features of cellulosic paper support the growing enthusiasm of µPADs. Paper has higher surface area to volume ratio and the microfiber network morphology has high loading capacity. The hydrophilic microfibers allow fluids and associated analytes to wick without any external force. The surface functionalization in this natural biocompatible polymer is well-established for attaching biomolecules. Paper microfiber network is suitable as a support for cells or microorganisms without causing adverse effects. The lateral flow assays are the early versions of μPADs that have been used for routine point-of-care diagnostics. The lateral-flow immunoassays (LFIA) such as those used for HIV detection, pregnancy, and drug tests are good examples of commercially available inexpensive rapid paper-based assays to detect disease biomarkers. The μPADs have been used in the quantitative analysis of a number of analytes in various areas, such as medicine, healthcare, and environmental monitoring. When compared to the conventional microfluidic analytical devices fabricated on silicon, glass, and superpolymer as substrates, the μPADs are low-cost, have simple fabrication processes, user-friendly, allow visible signal that is readable by eyes, and require less external instrumentations making them best suitable for resource-limited regions. [1] However, the growing demand for increased analytical sensitivity challenges the current format of μPADs assays, especially those involving colorimetric detection. Target biomolecules present at low concentrations require sample pretreatment, signal amplification, and detection methods to improve their limits of detection (LOD) and hence, enhanced sensitivity of μPADs becomes necessary so as to increase the power and wider applicability of μPADs in chemical- and bioanalyses.
The detection limits can be improved by increasing the signal to noise ratio of the assay. The signal can be increased either by preconcentrating target analyte molecules or the signaling molecules produced after the assay reaction or both. Even though several strategies have been explored for signal amplification on traditional microfluidic platform, especially for immunoassays [2], there have been only few efforts to improve the signal for μPADs. As this is a rapidly growing field, I expect more reports on novel ideas on increasing the sensitivity of μPADs methods coming in future. In this opinion piece, I discuss some of the major efforts that have been already made to improve the ability of paper-devices to detect trace concentrations of target analytes and future perspective in this area.
Improving detection limits on paper-devices
synthetic or natural based materials depending on their origin. Among the synthetic ones, molecularly imprinted polymers (MIPs) [10] and restricted access materials (RAMs) [11] can be highlighted. However, natural based materials comprising biomolecules are gaining special interest due to their enhanced selectivity (close to specificity) towards the target analytes [12]. Some of the biomolecules, like antibodies and enzymes, present selective cavities towards specific targets. Other biomolecules, like aptamers, can be in-vitro selected to bind the target in a very selective way [13].
The ideal microextraction technique in bioanalysis
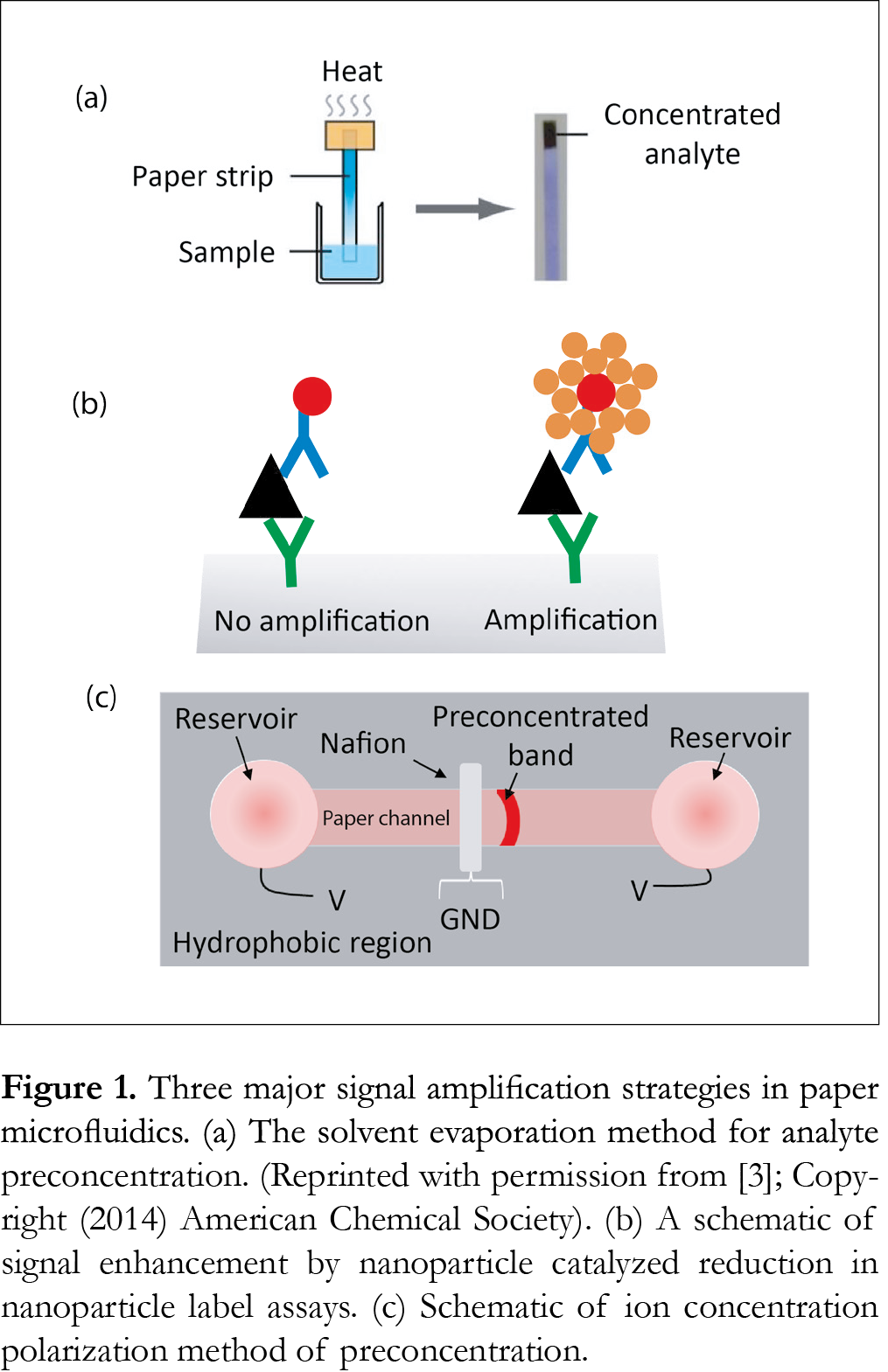
The major efforts reported so far to increase the signal involve evaporation of solvent, variation in geometry and dimensions of paper reservoirs and channels, employing nanoparticle as labels, and electrokinetic concentrators such as ion concentration polarization (ICP) and isotachophoresis (ITP) etc. (Fig. 1).
Wong et al. [3] have developed a simple evaporative concentration method on a paper-based device to concentrate tuberculosis-specific glycolipid, lipoarabinomannan in simulated urine. This technology relies on the application of localized heat to a paper strip to evaporate off hundreds of microliters of liquid to concentrate the target analyte by 20-fold in 20 min.
The use of nanoparticles and enzymes labels is not completely a new concept in bioanalysis, especially in immunoassays. Many commercially available LFIAs use nanoparticle labels for signal generation for colorimetric detection. But these are mainly qualitative or semi-quantitative. Recently, some efforts have been made to increase the signal from nanoparticle labels to enhance the sensitivities of bioassays, most notably, immunoassays. The focus of this article is towards the quantitative μPADs that actually enhance the signal of the assays on top of using nanoparticle labels. Cho et al. [4] adopted an immunogold–silver staining method, which combined immunological reactions and silver intensification in a cross-flow chromatographic analysis. The silver ions are reduced to elemental silver by gold nanoparticles that increases the signal generated from the assay. This method enhanced the detection capability by 51-fold compared to the conventional rapid test kit using 30 nm-sized colloidal gold as the tracer. In contrast to above example, Professor Paul Yager’s group at the University of Washington has used gold enhancement solution [5-7] to increase the size of nanoparticles used as label in paper network. BSA-biotin was spotted in the capture spot of the detection region and SA-gold solution was then added to measure the signal. The signal was then amplified by adding gold enhancement solution which deposited additional gold onto the pre-existing gold nanoparticles yielding an amplification of 5.9. They have [6] also demonstrated similar idea for detecting human chorionic gonadotropin (hCG) hormone yielding a LOD of 1 mlU/mL (4-fold improvement). Abbas et al. [8] reported a plasmonic paper-based analytical platform in which a star-shaped device was used along with gold nanorods and surface-enhanced Raman scattering as a transduction. This design generated a rapid capillary-driven flow capable of dragging liquid samples as well as gold nanorods into a 10th of micrometer size single cellulose microfiber. Accumulation of these molecules and nanorods resulted an extremely preconcentrated and optically active detection spot. The combination of this concept with surface-enhanced Raman scattering analysis yielded extremely low LODs (~100 aM for fluorescein and 500zM for naphtalenethiol). A single-step, paper-based concentration and detection of a malaria biomarker has been recently described by Pereira et al. [9], micellar aqueous two-phase surfactant system was applied to a 3-D paper design, which was integrated to LFIA to simultaneously concentrate and detect plasmodium lactate dehydrogenase (pLDH), a malaria biomarker. This method was able to give a LOD of 1.0 ng/µL in 20 min for pLDH compared to a conventional LFIA setup, demonstrating a 10-fold improvement.
Next area of preconcentration methods involve electrokinetics in which electric field is applied to separate and preconcentrated charged molecules. Electrokinetic techniques have been used in conventional microfluidic devices for several years. The feasibility of using electrokinetic concentrators in paper microfluidics has attracted little attention but is increasing in recent years. Gong et al. [10] recently integrated a nanoporous Nafion membrane (pore diameter ~ 4nm) into a paper strip to make an ICP-based concentrator. The paper-ICP system was able to preconcentrate a fluorescent tracer up to 40-fold. The same system improved the LOD of detecting dye tagged albumin by a factor of 5 compared to the case without ICP. ICP is an electrokinetic phenomenon caused by the transport of ions through nanostructures. It occurs at the interface of microfluidic and ion selective nanofluidic channels under an applied field. The ICP method has also been used in paper device to improve the detection sensitivity of deoxyribonucleic acid (DNA) [11]. Hepatitis B virus DNA targets in human serum were simultaneously preconcentrated, separated, and detected in a single 10 min operation resulting in a LOD of 150 copies/mL, which is sufficient for early diagnosis of hepatitis B. A low-cost approach for sample concentration using ICP was reported by Phan et al. [12]. The device fabricated using simple paper cutting lamination in which off-the-self Nafion membrane was integrated as nanoporous junction demonstrated an effective microfluidic concentrator giving a 60-fold signal enhancement in 200 s for a fluorescent dye. Similarly, Yang et al. [13] at first demonstrated the ICP phenomenon in a µPAD with a Nafion ion-selective membrane using fluorescein, which resulted in a 20-fold enhancement in 130 s with the application of an external potential of 50V. Later on same procedure was verified using a relatively more applicable molecule, the fluorescein isothiocyanate labeled bovine serum albumin that showed a 15-fold enhancement.
Another popularly used electrokinetic concentrator method is ITP. ITP is a nonlinear electrophoretic technique used to preconcentrate and separate a variety of ionic compounds. This preconcentration method has been shown to yield millions fold signal amplification in traditional microfluidic platforms. In ITP, sample ions focus between leading (LE) and trailing electrolytes (TE) with higher and lower effective electrophoretic mobilities, respectively than the sample ions. When a constant voltage is applied across the channel, sample ions preconcentrate into a narrow zone (~100 μm) between LE and TE zones. Moghadam et al. [14] used ITP preconcentration and separation technique to focus target analytes into a thin band and transport them to the LFIA capture line, resulting in a dramatic increase in the surface reaction rate and equilibrium binding. This ITP method was able to improve the LOD of LFIA by 400-fold in 90 s assay time. The LFIA on nitrocellulose membrane had labeled secondary antibody IgG as the target. The target antibodies were focused into a narrow ITP zone and transported them across the nitrocellulose membrane to the test zone. The high target extraction and preconcentration of ITP enables the LFIAs to use a large volume of sample when the target is very dilute, e.g., blood and wastewater. Rosenfeld et al. [15] presented an experimental and analytical study of a novel μPAD for isotachophoretic sample focusing. They demonstrated the processing of 30 μL of sample achieving 1000-fold increase in peak concentration in 6 min. In this case, Joule heating was overcome by designing the fabrication process such that a thick layer of wax deposited at the bottom of the channel enables the creation of shallow channels, resulting in sufficiently rapid heat dissipation. Although the dispersion is much more significant in paper than in glass, peak enhancement by substantial sample focusing was achieved in several minutes. Similarly, same principle of ITP [16] was applied to demonstrate about 900 fold preconcentration of Alexa Fluor 488 on a nitrocellulose membrane. These examples confirm that paper-based channels have similar electrokinetic properties to those of conventional PDMS/glass chan
Figures and Tables
[Click to enlarge]
Discussion and future perspectives
Paper as a platform for chemical and biological analyses has enormous potential. Its potential will rise with improvements in fabrication and analysis techniques in coming days. However, the sensitivity of the device appears to remain a frequently encountered limitation. Few papers published in past couple of years have shown very promising results and hope for increasing sensitivity of paper-based bioanalysis methods. Bioanalysis on paper-devices are known for their simplicity on fabrication and use, portability, are claimed to be best suitable for resource-limited and remote settings. Any efforts to enhance the sensitivity of these assays should not compromise on their above fundamental values. The major efforts discussed in this opinion ranged from solvent evaporation and gold amplification of nanoparticle label signals to electrokinetic methods. While non-electrokinetic methods discussed here do not involve extra instrumentations, most of the electrokinetic methods require electric field to induce sample flow and preconcentration and thus require additional instrumentation for power supplies and fabrication steps. In addition, the electrokinetic-based methods work only for charged samples. Future efforts may come up with small button battery powered μPAD electrokinetic systems to make the devices portable suitable for remote places. Once a paper-based assay is saturated, further analyte transport is generally not possible limiting the capture and thus concentration of more target molecules. The electrokinetic techniques can achieve post-wetting manipulation. Further, ITP on paper has the ability to process large sample volumes that can be utilized to analyze dilute but large sample volumes. The electrochemical detection in paper-device are also widely used but need to have amplification strategies to increase the sensitivity in addition to incorporating portable and hand-held external power and potentiostat [17]. Sensitive paper-analytical methods have great potential in future. This technology potentially could transform the current state of diagnostic assays for various diseases such as malaria, human immunodeficiency virus, tuberculosis, especially, in resource-poor settings, ultimately saving millions of lives and on field testing of environmental pollutants.
References
1. Cate DM, Adkins JA, Mettakoonpitak J, Henry CS. Recent developments in paper-based microfluidic devices. Anal Chem 87(1), 19-41 (2015). [CrossRef]
2. Giri B, Pandey B, Neupane B, Ligler F. Signal amplification strategies for microfluidic immunoassays. TrAC. http://dx.doi.org/10.1016/j.trac.2015.10.021.
3. Wong SY, Cabodi M, Rolland J, Klapperich CM. Evaporative concentration on a paper-based device to concentrate analytes in a biological fluid. Anal Chem. 86(24), 11981-11985 (2014). [CrossRef]
4. Cho IH, Seo SM, Paek EH, Paek SH. Immunogold–silver staining-on-a-chip biosensor based on cross-flow chromatography. J Chromatogr B Biomed Appl. 878(2), 271-277 (2010). [CrossRef]
5. Fu E, Kauffman P, Lutz B, Yager P. Chemical signal amplification in two-dimensional paper networks. Sensor Actuat B-Chem. 149(1), 325-328 (2010). [CrossRef]
6. Fu E, Liang T, Houghtaling J, Ramachandran S, Ramsey SA, Lutz B, et al. Enhanced sensitivity of lateral flow tests using a two-dimensional paper network format. Anal Chem. 83(20), 7941-7946 (2011). [CrossRef]
7. Fridley GE, Le H, Yager P. Highly Sensitive immunoassay based on controlled rehydration of patterned reagents in a 2-dimensional paper network. Anal Chem. 86(13), 6447-6453 (2014). [CrossRef]
8. Abbas A, Brimer A, Slocik JM, Tian L, Naik RR, Singamaneni S. Multifunctional analytical platform on a paper strip: separation, preconcentration, and subattomolar detection. Anal Chem. 85(8), 3977-3983 (2013). [CrossRef]
9. Pereira DY, Chiu RY, Zhang SC, Wu BM, Kamei DT. Single-step, paper-based concentration and detection of a malaria biomarker. Anal Chim Acta. 882, 83-89 (2015). [CrossRef]
10. Gong MM, Zhang P, MacDonald BD, Sinton D. Nanoporous membranes enable concentration and transport in fully wet paper-based assays. Anal Chemistry. 86(16), 8090-8097 (2014). [Crossref]
11. Gong MM, Nosrati R, San Gabriel MC, Zini A, Sinton D. Direct DNA analysis with paper-based ion concentration polarization. J Am Chem Soc. 137(43), 13913-13919 (2015). [CrossRef]
12. Phan DT, Shaegh SAM, Yang C, Nguyen NT. Sample concentration in a microfluidic paper-based analytical device using ion concentration polarization. Sensor and Actuat B-Chem. 222, 735-740 (2016). [CrossRef]
13. Yang RJ, Pu HH, Wang HL. Ion concentration polarization on paper-based microfluidic devices and its application to preconcentrate dilute sample solutions. Biomicrofluidics. 9, 014122 (2015). [CrossRef]
14. Moghadam BY, Connelly KT, Posner JD. Two orders of magnitude improvement in detection limit of lateral flow assays using isotachophoresis. Anal Chem. 87(2), 1009–1017 (2015). [CrossRef]
15. Rosenfeld T, Bercovici M. 1000-fold sample focusing on paper-based microfluidic devices. Lab on a Chip. 14, 4465–4474 (2014). [CrossRef]
16. Moghadam BY, Connelly KT, Posner JD. Isotachophoretic preconcenetration on paper-based microfluidic devices. Anal Chem. 86(12), 5829-5837 (2014). [CrossRef]
17. Maxwell EJ, Mazzeo AD, Whitesides GM. Paper-based electroanalytical devices for accessible diagnostic testing. MRS Bull. 38(04), 309-314 (2013). [CrossRef]
All site content, except where otherwise noted, is licensed under a Creative Commons Attribution 4.0 License